MOJ
eISSN: 2471-139X


Research Article Volume 4 Issue 4
Department of Physiology, Little Flower Medical Research Centre, India
Correspondence: Mukkadan JK, Research Director, Department of Physiology, Little Flower Medical Research Centre LFMRC, Angamaly, Kerala, India, Tel 91 9387518037, Fax 0484 2452646
Received: October 06, 2017 | Published: November 10, 2017
Citation: Devi NP, Mukkadan JK. Impact of rotatory vestibular stimulation in memory boosting. MOJ Anat Physiol. 2017;4(4):337–342. DOI: 10.15406/mojap.2017.04.00143
Objective: To find out the effect of rotatory vestibular stimulation on cognition in rats through examining the behavioral patterns, the alterations in dendritic arborization and changes in AChE activity.
Methods: Rotatory vestibular stimulation was provided in a rotatory vestibular apparatus at a rate of 50rpm for 5minutes, for 30days for rats. 0.3mg/kg of physostigmine also administered to rats of another group as a standard drug. No rotatory vestibular stimulation or physostigmine is given to the control rats. Behavioral analysis, Neuromorphological and Biochemical studies were done after vestibular stimulation.
Results: Number of trails for acquisition and retention reduced significantly in treated rats when compare with the control rats. In all the treated rats the dendritic arborization increased significantly and activity of AChE decreased significantly when compare with the control.
Conclusion: Rotatory vestibular stimulation enhances learning and memory via increasing dendritic arborization and inhibiting acetylcholinesterase activity in rats.
Keywords: rotatory vestibular stimulation, learning, memory, hippocampal pyramidal neurons
Phy, physostigmine; RVS, rotatory vestibular stimulation
Learning a behavioral task or something complicated with excess physical activity causing the subjects to shift away from homeostasis. This result in consumption of more energy leads to physiological stress. This in turn leads to the production of free radicals which will lead to oxidative stress. This can be harmful for proteins or DNA involved in dendritic growth. This will get reflected in learning and memory performance also. Enriched environments and soothing physical activities can be a good remedy for memory problems and also to enhance learning and memory. Different Types of Environmental Enrichments are in the following Table 1.
Vestibular Stimulation - A method of Environmental Enrichment! Improves Cognition (Figure 1).
Physical enrichments |
Exercises and physical activities, jumping, swimming, swinging |
Nutritional enrichments |
Various plant resources and rasayanas |
Social enrichments |
Peer groups and social activities |
Novel objects and accessories |
Visual, auditory and olfactory |
Colorful toys, tunnels and ladders, hanging objects |
Table 1 Various types of environmental enrichments.
How Vestibular system linked with hippocampus
The major anatomical pathways through which the vestibular signals reach the hippocampus is represented in Figure 2.
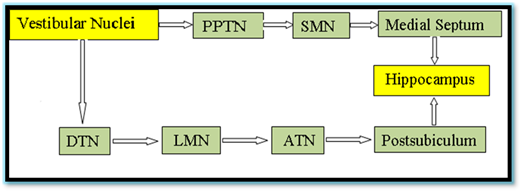
Figure 2 The major anatomical pathways through which the vestibular signals reach the hippocampus
PPTN: Pedunculopontine Nucleus, SM: Supramammilary Nucleus, Medial Septum; DTN: Dorsal Tegmental Nucleus, LMN: Lateral Mammilary Nucleus; ATN: Anterodorsal Thalamic Nucleus
Vestibular stimulation can be considered as the input that our body receives when we experience movement or gravity. Vestibular Stimulation can be,
Rotatory chairs predominantly activate the semicircular canals of the inner ear helps in the retention of memory.1
Animals used for the study
Male Wistar albino rats of 30days old weighing about 120±30gm were used in the study.
Rotatory vestibular apparatus
A new instrument has been designed in our laboratory for providing vestibular stimulation by rotations to find out whether learning and memory can be enhanced in rats through rotatory vestibular stimulation. This instrument was designed out of fiber frame as the basement with three plastic cages attached on it to place rats. The cages were of about 15cm length and 10cm width. Only one animal can occupy comfortably in one cage without any entrapment stress. The device works on electricity and the movement provided by this device gives vestibular stimulation in rotation mode. The speed of rotation was 50 revolutions per minute in clock wise direction were fixed by trial and error method with 25rpm and 75rpm.2 Schematic Representation of Rotatory Vestibular Stimulating Apparatus is given in Figure 3.
Experimental design
Rats were randomly divided into different groups comprising 18 rats. Physostigmine (Standard drug) and Rotatory Vestibular Stimulation were administered to group B and C respectively for 30days before the beginning of the behavioral task and also 15minutes prior to the start of acquisition phase as well as each retention test.
Group A: Control group (Neither Vestibular Stimulation, nor the drug was administered).
Group B: Standard drug Physostigmine Treated Group (Rats of Group B were administered with the standard drug Physostigmine, 0.3mg/kg intraperitoneally)
Group C: Rotatory Vestibular Stimulated Group (RVS).
Experimental design for behavioral analysis and administration of rotatory vestibular stimulation and physostigmine: After 30days of vestibular stimulation, the rats were subjected for Behavioral studies in Radial arm Maze. The behavioral experiments were carried out in three phases, viz; Orientation and Training Session, Learning Performance Test (Acquisition Test), and Memory Performance Test (Retention test). The rats were semi starved for 48hrs. Before the start of behavioral experiments. During the threedays of orientation the semi starved rats were allowed to familiarize themselves with the radial maze. After the orientation phase, the behavioral task was performed, where all the eight arms of the maze were baited with food pellets (Kellogg’s chocolate wheat scoops) and then the rat was placed in the center of the maze and allowed to freely explore the maze. The rats were required or trained to take the food pellet from each arm without making a reentry in to the already visited arm. The training or trial was terminated when the animal learned to take the food reward from the all eight arms or after 10 minutes if all the eight arms were not visited. Six trials per day was given with an inter trial interval of 1hour. To avoid olfactory cues, the maze was wiped with 70% ethanol prior to each session.3 After acquisition phase all the trained rats were kept for consolidation of the learned task for 10days. After 10days of acquisition, the retention test was carried out until the rats attaining the learning criteria.4,5 For the assessment of learning and memory the no. of trails taken for attaining the task were recorded. For analyzing the Long Term Potentiation (LTP), the retention test was repeated for 7times with 10days of gap in between each test.6 Schematic Representation of Eight Arm Radial Maze is given in Figure 4.
Control rats (Group A) were under gone the same procedure of behavioral task without providing any drug or vestibular stimulation. The rats of Group B were administered with the drug physostigmine (0.3mg/kg, orally)7,8 for 30days without any vestibular stimulation and kept as standard drug group.
Neuromorphological analysis
After the behavioral studies Neuromorphological analysis were carried out. The brain was shelled out and the hippocampus dissected out for processing with Rapid Golgi staining method.9,10 After staining, the hippocampal sections were taken at 120µm thickness using rotary microtome and Camera lucida tracings of Hippocampal pyramidal neurons were done. Dendritic branching points and intersections were counted by Sholl Analysis Method.
Acetylcholinesterase activity
AChE activity was done by using UV Spectrophotometer by Ellman et al.11 method. Immediately after last retention test, the rats were sacrificed and the hippocampus was dissected out in an ice cold 0.1M phosphate buffer saline (pH 7.2). The hippocampus (10mg/ml) was homogenized in ice cold 0.1M phosphate buffer saline (pH 7.2) using Teflon homogenizer. The homogenate were centrifuged at 5000rpm for 10min at 40C. The supernatant (0.4ml) mixed with 2.6ml of phosphate buffer (0.1M, pH 8) and 0.1ml of DTNB (0.01M). After that 0.1ml of acetylthiocholine iodide added, the absorbance was measured every one minute for 10minutes at 412 nm using Spectrophotometer
Statistical analysis
Statistics were done using Graph pad prism (5.0) software. Results are shown in Mean±SD. ANOVA followed by Tukey’s multiple comparisons post hoc is done in the study.
Behavioral analysis
The effect of Rotatory Vestibular Stimulation on learning and memory was investigated in rats using Radial Arm Maze task. The acquisition and retention of the three groups were statistically analyzed using one way ANOVA followed by Tukey’s multiple comparison tests.
Acquisition: The mean no. of trails for acquisition in Group B (8.83±0.86, p<0.001) and Group C (8.17±1.69, p<0.001) shows a significant reduction when compare with Group A (30.44±1.15). But there is no significant difference between Group B and Group C indicates that Rotatory vestibular stimulation and physostigmine are equally good at enhancing learning capacity. Results shown in Table 2.
Groups (n=18) |
Number of Trails (Mean±SD) |
Group A Control |
30.44±1.15 |
Group B Phy |
8.83±0.86a |
Group C RVS |
8.17±1.69a,ns |
Table 2 The Number of trials taken by each group of rats in Acquisition
Phy: Physostigmine; RVS: Rotatory Vestibular Stimulated
Retention-memory from the 10th- 70st day after acquisition
From the analysis, it is observed that, the treated Groups (B and C) shows significant decrease in number of trials taken for both acquisition and retention when compared with the Control (Group A), but no significant difference was observed between the Group B (Physostigmine) and Group C (RVS). In retention from the 50th day of retention onwards a considerable reduction in number of trails were seen within each treated groups. This indicates that Rotatory Vestibular stimulation and physostigmine equally responsible for LTP.
Neuromorphological analysis
Dendritic branching points: 0-20 µm concentric circle: The treated Groups B (10.30±0.63, p<0.01) and C (13.63±0.99, p<0.001) shows significant increase in number of branching points when compared with Group A (7.33±0.92). There is a significant increase in dendritic branching points in Group C (p<0.01) when compare with Group B.
From the analysis it is clear that dendritic branching points are significantly increased in 20-40 µm to 80-100 µm concentric circles, when compare with the control and among these 20-40µm concentric circle shows a highly increased branching points in comparison with others. There is a significant increase in Group C than Group B indicates that Rotatory Vestibular Stimulation enhances alters the dendritic branching points. Results shown in Figure 6.
Dendritic intersections
From the analysis it is clear that the dendritic intersection increased from 40 µm concentric circle to 80µm concentric circle, in each group but dendritic intersection gradually decreased in 100 µm and 120µm. Dendritic intersection at its peak was observed in 80 µm. Results shown in Figure 7.
It is clear from the neuromorphological analysis that dendritic arborization is increased in Group B and C when compare with the Control Group, but Group B and C shows a rather similar result in dendritic intersection (Figure 8) .
Biochemical analysis
Rate of Acetylcholinesterase of Group B (5.17 ±0.71) and C (5.24 ±0.55) is significantly decreased when compare with the Group A (6.87 ±0.65, p< 0.001). There is no significant difference between group B and C. From the result it is clear that rate of AChE activity is reduced in treated groups of rats and this in turn results in an improved learning and memory. Results shown in Figure 9.
In the present study rats provided with RVS enhanced learning and memory through increasing dendritic arborization and decreasing AChE activity. Comparable results were observed with the standard drug also. This indicates that Rotatory Vestibular Stimulation enhances long term potentiation (LTP) as physostigmine improves memory. This is in par with several earlier reports.12 Different types of rotations provides vestibular stimulation are, spinning, dancing, rolling, jumping, running, soccer and basketball games, rotating circular, or turning types of motion. Stimulation at the maximum happens when the movement is started or stopped or where there is a change in the speed of the motion. Slight stimulation has occurred in between starting and stopping. Head rotations result stimulation of the semicircular canals by the movement of the fluid in the semicircular canal. Stimulation of hippocampal neurons holds back glucocorticoids secretion, a hormone which impairs learning and memory.13 Still, a common disparagement to spinning motion14and centrifugation studies15 is that both engross combined semicircular canal and otolith stimulation. Accordingly the exact input of the semicircular canal to the acuity of verticality cannot be clearly comprehended. But in the present study vestibular stimulation in a comfortable speed diminishes stress and also stimulates the semicircular canal and made learning and memory easy. Vestibular nuclei and hippocampus have anatomical connections16 and vestibular stimulation hinder both the stress axes (HPA- hypothalamic-pituitary-adrenocortical) and sympathetic adrenomedullary (SAM) axis, and consequently decreases glucocorticoids and cortisol level and also maintains heart rate and blood pressure within normal range, promotes sleep, and thus improves cognition.17‒19 This may be because of the firing of two types of hippocampal neurons (place cells and HD cells) crucial for spatial behavior through vestibular stimulation.20 Vestibular stimulation is a very effectual and dependable loom for treating attention deficit or hyperactivity disorder particularly when combined with other training because vestibular stimulation activates the hippocampal formation, parietal cortex and retrosplenial cortex in humans21 and rats.22In addition vestibular stimulation alters primate neuronal activity and rat hippocampal place cell activity.23,24
The hippocampal theta rhythm and HD cell activity also augmented through vestibular input.24,25 The horizontal semicircular canals activated and stimulated by rotation. Rotatory chairs activate particularly the horizontal semicircular canals when the participant is in upright sitting position. Behavioral reactions may be multifarious in the centrifugal rotation. The centrifugal rotations induce a combined tilt-translation sensation such as being on a gondola. These results suggests to the use of linear motion stimulators allowing the motion and positioning in space to stimulate the otolithic organ, and also frequency and displacement range is rationally limited when compared to rotatory chairs. Hippocampus is the fundamental integrative center which regulates the exploratory activities and also for incorporating spatial information.26 An increase in the dendritic arborization and synapses in the hippocampal pyramidal neurons results in the facilitation of cognition (learning/acquisition) and performance in the spatial learning tasks. The increase in dendritic arborization also increases the number of synaptic connections with the neurons. This can be considered as the root of neural basis for the improved cognitive functions in the treated rats. In the present study we used rotatory vestibular stimulation and noticed significant improvement in cognition as the horizontal semicircular canals activated by rotation and also the hippocampal formation acquired activation and increased dendritic arborization and decreased AChE activity without any discomfort or physical illness in rats.
Learning and memory can be enhanced by rotatory vestibular stimulation via increased dendritic arborization and decreased AChE activity. Rotatory vestibular stimulation can be recommended as a safe and unpretentious way of improving learning and memory power. Further studies have to be done to find out the BDNF level in vestibular stimulated rats.
We acknowledge the kind help given by Fr. Sebastian Kalappurackal, Director, Little Flower Hospital and Research Centre for carrying out this research work at Little Flower Medical Research Centre, Angamaly.
There is no conflict of interest in this research work.

©2017 Devi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.