MOJ
eISSN: 2471-139X


Research Article Volume 7 Issue 5
1Department of Medicinal Chemistry, Faculty of Pharmacy, University of Tripoli, Libya
2Novelien School, Tripoli Centre, Libya
3National Commission for DNA Research and Analysis, Libya
4Food and Drug Control Centre (LFDA), Libya
5Zoology Department, Faculty of Science, Tripoli University, Libya
6Department of Biosciences, University of Salzburg, Austria
Correspondence: Abdul M Gbaj, Professor of Genetics and Biochemistry, Department of Medicinal Chemistry, Faculty of Pharmacy, University of Tripoli, Libya, Tel 00218913556785, Fax 00218213405023
Received: August 16, 2020 | Published: September 22, 2020
Citation: Sadawe IA, Gbaj AA, Algheryane AO, et al. Evaluation of aerial parts of Echium angustifolium on ciguatoxins toxicity using molecular modeling and albino mice models. MOJ Anat Physiol. 2020;7(5):134-145. DOI: 10.15406/mojap.2020.07.00304
Ciguatoxin (CTX) is a polyether neurotoxin compound produced by microalgae (dinoflagellate, Gambierdiscus spp). The toxin is accumulated and transformed throughout the sea food chain causing many life-threatening neurological problems in Libya and other Mediterranean and North African countries and eventually may cause death.
The plant species Echium angustifolium which is locally known as “Hannet Al-Aggrab” contains pyrrolizidine alkaloids, phenolic acid derivatives, flavonoids and other constituents that are known for their numerous biological activities. For treatments of ciguatera fish poisoning the plant has not been studied so far in the Mediterranean and North Africa regions. In the present study, Echium angustifolium aqueous extract was evaluated for its ability to reduce or revoke the effect of ciguatoxins in mice. Molecular docking and in vivo animal studies were performed in order to determine the potential effect of Echium angustifolium aqueous extract against ciguatoxicity. The content of Echium angustifolium extract was evaluated using molecular modeling against ciguatoxin on sodium (Na+) and potassium (K+) voltage-gated channels. Hesperetin was found the most active compounds with a Gibbs energy of -8.5Kcal/mol. For K+ voltage-gated channels was ellagic acid which was the most active compounds with a Gibbs energy of -9.0Kcal/mol. Our results revealed that Echium angustifolium constituents form hydrogen bonds with active sites of Na+ and K+ channels and protect the mice from ciguatoxin toxicity with a statistically significant difference of the extract compared to controls with p value less than 0.01. We propose that Echium angustifolium constituents can be used to prevent ciguatoxin toxicity. Further chemical synthesis of analogues and in vivo studies will be necessary to substantiate the obtained results.
Keywords: ciguatera fish poisoning, ciguatoxin, neurotoxin, molecular modeling, Na+, k+ channels
Ciguatera Fish Poisoning (CFP) which causes considerable physical and functional impact in humans is the most often reported seafood intoxication worldwide. Ciguatera toxins are lipid soluble, heat stable cyclic polyethers produced by dinoflagellate of the genus Gambierdiscus toxicus contained in the marine benthic algae plankton.1–3 The microalgae are consumed by herbivorous fish along with macrophytic algae on which it resides. The toxins are passed on to the food chain and after they reach an adequate concentration they incite human poisoning known as ichthyosarcotoxism. Contaminated fish cannot be recognized by odor, appearance or taste and therefore is hard to avoid. All species of fish linked with coral reefs could be toxic, particularly those at the upper end of the food chain (seaperch, groupers, barracudas, sharks, moray eels, etc).4–6
Numerous ‘ciguateric’ toxins, are reported being involved in the etiology of ciguatera fish poisoning have been reported. The two major toxins are ciguatoxin (CTX) and maitotoxin (MTX).7,8 CTX which cannot be damaged by freezing or cooking is a powerful marine toxin with a fifty percentage lethal dose (LD50) in mice of 0.45µg/kg (i.p.).9,10 Maitotoxin, which can be biosynthesized in cultures of Gambierdiscus toxicus, is even more toxic (LD50=0.13µg/kg, i.p.). Ciguatoxin and maitotoxin are initially made by a small marine organism, Gambierdiscus toxicus that grow on and around coral reefs in subtropical and tropical waters. Gambierdiscus toxicus are eaten by herbivorous fish which in turn are eaten by larger carnivorous fish and both toxins become more concentrated as they move up the food chain.11 Ciguatera fish poisoning has a polymorphous emergency whose symptoms of poisoning appear 2-30hours and are characterized by a wide range of cardiovascular, gastrointestinal (GI), and neurological symptoms which include vomiting, nausea, diarrhea, ataxia, joint pain, reversal of temperature sensations, coma, and death.12,13 The gastrointestinal symptoms frequently start within twenty four hours after consuming fish that contain ciguatera toxins. Even though mortality is low approximately two percent, total recovery characteristically takes from a few days to one week in mild intoxications and from several weeks to months and even years in harsh attacks.14 In addition to the availability of a specific immunological technology for assessment of toxins in seafood products, the clinical diagnosis helps the recognition of people that ingested toxic fish.14,15 The pathophysiological effects of ciguatoxins are defined by causing continual activation of neuronal voltage-sensitive Na+ channels and inhibition of K+ channels, leading to neuronal excitability, increased neurotransmitter release as well as impaired synaptic vesicle recycling, elevation of intracellular calcium ion concentration and in addition causing edema of axons and Schwann cells leading to spontaneous and repetitive action potentials.16,17
Echium angustifolium (family: Boraginaceae) a wildflower growing in the Mediterranean regions such as Libya, Algeria, Tunisia, Greek, etc. The plant contains Allantoin and pyrrolizidine alkaloids (i.e. Heliosupin), phenolic acid derivatives, flavonoids and other constituents which are known for their numerous biological activities and it is weakly poisoning for small warm-blooded animals. It is not dangerous for humans and sheep neutralize the active ingredients in their stomach. In small doses this medical plant is used as diuretic, anti-inflammatory, astringent or antirheumatic. However, after prolonged ingestion it may cause liver damage or will be carcinogenic.18,19
Molecular docking and in vivo animal studies were performed in order to determine the potential effect of the plant extract. Molecular docking results can give information that can be used to guide and develop an array of experiments.20,21 Among all the molecules that were evaluated using molecular modeling against ciguatoxin, hesperidin on Na+ voltage-gated channels and ellagic acid and on K+ voltage-gated channels were investigated. The achieved results revealed that Echium angustifolium constituents form hydrogen bonds with active sites of Na+ and K+. In the present study an aqueous extract of Echium angustifolium was evaluated for its ability to reduce or revoke the effect of ciguatoxins in mice. Interaction of aqueous extract of Echium angustifolium with ciguatoxins may lead to the neutralization/inhibition of the ciguatoxins activities.
Collection of plant material and preparation of aqueous extract
Plants were collected from the Garabolle Zone, Tripoli, Libya (March 2020), and Echium angustifolium was identified and authenticated by a botanist. The sample was initially rinsed with distilled water and dried at room temperature. The leaves with the stems were cut into smaller pieces and 1.29g of the sample was taken. The cut leaves and stems were then grinded in a homogenizer (HO4A Edmund Buhler GmbH, UK) along with 30ml of distilled water. The resulting aqueous solution was filtered under vacuum using a Millipore filter (0.45μm, GHD Acrodisc GF, UK) and the filtrate was stored at 4°C.
Sampling and toxin extraction
Specimens of fish (n=6) Sarpa salpa, commonly known commonly as the dreamfish, salema salema, cow bream, porgy or gold line were used for experiments. The fishes were caught at different locations on the Tripoli-Libya coast during January 2020. Immediately following the collection, the fishes were eviscerated and frozen at -20°C until use. In brief, one gram from each organ [(flesh (including muscles and skin), liver, brain and viscera)] was homogenized with 5ml of 0.1% acetic acid. The samples were boiled in a water bath for 10min at 50°C and then cooled to room temperature. The samples were centrifuged at 3000 RPM for 10 minutes at 10°C. The obtained supernatant from specimens was collected. Each aliquot was conserved at -20°C until further use.
Molecular docking
The starting geometry of the Echium angustifolium components were constructed using chem3D Ultra (version 8.0, Cambridge soft Com., USA). The optimized geometry with the lowest energy was used for molecular docking. Crystal structures of voltage-gated Na+ (6AGF, Nav1.4) and K+ (1BL8, KcsA) channels in a complex with transition-state analogues were downloaded from the Protein Data Bank https://www.rcsb.org/structure/6AGF and https://www.rcsb.org/structure/1BL8 for sodium and potassium channels, respectively (Figure 1). Molecular dockings of Echium angustifolium components with 6AGF and 1BL8 was accomplished by Auto Dock 4.2 software from the Scripps Research Institute (TSRI) (http://autodock.scripps.edu/). Firstly, polar hydrogen atoms were added into protein molecules. Then, partial atomic charges of 6AGF and 1BL8 and Echium angustifolium components were calculated using Kollman methods.22 In the process of molecular docking, the grid maps of dimensions (62Å X 62Å X 62Å) with a grid-point spacing of 0.376Å and the grid boxes centered were used. The number of genetic algorithm runs and the number of evaluations were set to 100. All other parameters were default settings. Cluster analysis was performed on the results of docking by using a root mean square (RMS) tolerance of 2.0Å, which was dependent on the binding free energy. Lastly, the dominating configuration of the binding complex of Echium angustifolium components and 6AGF and 1BL8 channels fragments with minimum energy of binding were determined which relied strongly on the information of 3D structures of 6AGF and 1BL8 ion channels binding sites and ultimately generated a series binding complexes, respectively (Figure 1).
Experimental models
Albino mice (Swiss type) of either sex weighing 18–28g (2 to 2.6 month old) were utilized for investigations. They were kept in cages made from polypropylene in an air-conditioned room at a temperature of 25±2°C, at a twelve hour dark/ light cycle. The mice were supplied with drinking water ad libitum and an adequate diet. Authorization for the experimental procedures was obtained from the Animal Ethics Committee from the National Research Centre, Zawia, Libya.
Acute toxicity study
Acute toxicity studies were performed to determine the LD50 value of experimental animals. The intend of performing acute toxicity studies was establish the therapeutic index of Echium angustifolium and to guarantee in-vivo safety. For male mice were randomly allocated into four groups (n=5). The first group served as control and was given 0.9% normal saline orally at 0.2ml/kg body weight. The remaining groups were given a single oral dose of either 50, 100, 400 or 800mg/kg body weight Echium angustifolium extract, respectively.23,24 Similar acute toxicity studies were performed for the flesh (keletal muscles and fat), liver, brain and viscera extracts. Acute toxicity experiments were also performed with twenty male mice randomly allocated into five groups (n=5). The first group served as control and was given 0.9% normal saline orally at 0.2ml/kg body weight. The remaining four groups were given a single oral dose of either 50, 100, 300 or 400µl of the flesh extract (1g/5ml 0.1 acetic acid). A similar protocol was used for liver, brain and viscera extracts.
Detoxication of ciguatoxins by Echium angustifolium extract
After acclimatization to laboratory conditions for 1 week, the animals (albino mice) used in this study were divided into five groups, each with six mice each (male or female). The first group received only two hundred microliter (1.0g/5ml) of the extract (LD50 0.45μg/kg). Groups 2 to 5 were given an equivalent amount extract with 100μl, 200μl, 300μl or 400μl of aqueous Echium angustifolium extract orally (1.29g/30ml), respectively. The number of death was recorded within twenty-four hours. Similar experiments were repeated with liver, brain and viscera extracts using groups 6 to 20.
In silico toxicity Assessment of Echium angustifolium components
The in silico toxicity assessment of all Echium angustifolium chemical components was made with online tool called ProTox-II: a webserver for the prediction of toxicity of chemicals ( http://tox.charite.de/protox_II/).25 The drug-likeness for Echium angustifolium components was evaluated through Lipinski Rule of Five using the server called Swiss ADME provided in the web link ( http://www.swissadme.ch/index.php ).26
The difference among various treated groups and control group were analyzed using one-way-ANOVA followed using unpaired Student’s t test. The results were expressed as the mean ± SEM of the number of experiments done, with P<0.05 indicating the significant difference between groups.
Acute toxicity study of Echium angustifolium aqueous extract (1.29g/30ml) given to albino mice
An Echium angustifolium aqueous extract was found to be safe up to 140mg/kg orally within two weeks. The present study is compared to other previous studies of the Boraginaceae plant family in which an intravenous single dose of 561mg/kg of rosmarinic acid did not produce acute toxicity in mice.27
Acute toxicity of Sarpa salpa extracts and its neutralization by Echium angustifolium aqueous extract
Crude ciguatoxin (neurotoxins) Extracts 1g/5ml 0.1% acetic acid LD50g/kg (orally) Protective dosage of Echium angustifolium aqueous extract (1.29g/30ml) in µl given to Albino mice
The Sarpa salpa toxin of four different tissues produced different LD50 values as shown in Table 1. The mice affected by the toxin exhibited typical signs of neurotoxic disorders including hypothermia, considerably reduced locomotor activity during the first 3 hours and eventually breathing failure. Table 1 shows a significant difference in toxicity between the four extracts. Clearly the concentrations of toxins in organs were different and can be ranked in ascending order: flesh, brain, liver, and lastly the viscera extract. The results are similar to the results obtained by Elfeki et al.28,29 The Echium angustifolium aqueous extract significantly increases mean survival time up to 5±1 days and protects animals from death if compared to the mice who had Sarpa salpa toxin only. Echium angustifolium aqueous extract if used at a higher doses was found to be more effective against Sarpa salpa toxin.
Crude ciguatoxin (neurotoxins) Extracts 1g/5ml 0.1% acetic acid |
LD50g/kg (orally) |
Protective dosage of Echium angustifolium aqueous extract (1.29g/30ml) in µl given to Albino mice |
Flesh extract |
51±2.6 |
125±2.3 |
Brain extract |
33±3.4 |
220±6.3 |
Liver extract |
16±2.6 |
302±7.4 |
Viscera extract |
8.2±1.1 |
411±11 |
Table 1 Protective effect of Echium angustifolium aqueous extract against flesh, brain, liver and viscera extracts of Sarpa salpa. Values are mean ± SEM of six animals. The LD50 shows a statistically significant difference of different extracts compared to controls (p value <0.01).
A Similar study was performed using H. foertherianum aqueous extract containing as major compounds rosmarinic acid which is able to reverse the P-CTX-1B-induced cytotoxicity on mouse neuroblastoma cells.27 The cytotoxicity obtained by P-CTX-1B was inhibited by H. foertherianum at concentrations up to 2734μg/ml and by rosmarinic acid up to 607μg/ml, concentrations at which they started to become cytotoxic.27 The toxicity study was performed by three cell viability methods: the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, a colorimetric assay for assessing cell metabolic activity and it reflects the number of viable cells present), LDH assays (lactate dehydrogenase assay for cell testing viability testing using cell lines and primary cultured astrocytes) and neutral red (a cytotoxicity assay used to detect cell viability or drug cytotoxicity based on the detection of viable cells via the uptake of the dye neutral red). The obtained results for H. foertherianum by Rossi et al.27 clearly demonstrate its ability to treat Ciguatera Fish Poisoning are consistent with our results. On the other hand; pharmacological tests are essential in order to decide whether or not Echium angustifolium indeed have direct ‘detoxifying’ action on the ciguatoxin itself in human and that is our program’s goal to assess the therapeutic potential of the Echium angustifolium aqueous extract in human.
Molecular docking analysis
The Na+ channel used for molecular docking was voltage-gated Na+ channel (denominated Nav1.4), which is responsible for action potential generation and is implicated in numerous human diseases. The model building was prepared for the pore domain, the voltage-sensing domains of the α-subunit and for β1 subunits, providing the molecular basis for Na+ ion penetration and kinetics.30 The K+ channel (denominated KcsA) used was from Streptomyces lividans and is a fundamental membrane protein with sequence similarities to many recognized K+ channels. K+ channels consist of four separate units linked together forming the ion pore where the chief chain carbonyl oxygen atoms are held unlock and provide an ion filter by structural constraints to organize passing K+ but not smaller Na+ ions. This arrangement endorses ion conduction by utilizing electrostatic repulsive forces to overcome attractive forces between K+ ions and the chief chain carbonyl oxygen atoms and helps in molecular docking of Echium angustifolium constituents. The construction of the pore creates the predominant selectivity toward potassium ions.31
Voltage-gated Na+ channels are important factors for the generation and propagation of electrical signals in the majority excitable cells. They are large membrane-spanning single proteins containing the ion pore which creates the rapid and transient increase in membrane Na+ ion conductance which is responsible for the depolarising part of action potentials. Persistant channel activation leads eventually to depolarization block and inhibition of action potential discharge.32,33 Ciguatoxin at low nanomolar concentrations induces spontaneous action potentials that can be suppressed TTX (Tetrodotoxin: is a specific Na+ channel blocker) indicating that this effect was mediated through voltage-gated sodium channels. Suppression of action potentials prevents nerve cells from carrying messages and thus inhibits muscles from contracting in response to nervous stimulation.32,33 Echium angustifolium constituents can reverse this effect. There are four main classes of potassium channels: calcium-activated potassium channel, inwardly rectifying potassium channel, tandem pore domain potassium channel and voltage-gated potassium channel and KcsA used in this study is more closely related to voltage-gated potassium channels.34,35 It has been reported that ciguatoxin also alters neuronal excitability by blocking of K+ channels contribute to membrane hyperpolarization. The block of voltage-gated K+ channels results in membrane depolarization caused by activation of voltage-gated Na+ channels. In addition, the block of K+ channels also contributes to a lowering of the action potential threshold due to the absence of the hyperpolarizing force caused by the K+ conductance.36 Both effects of ciguatoxin on voltage-activated Na+ and K+ channels can be reversed by Echium angustifolium constituents. Table 2 shows the binding energies of Echium angustifolium constituents to voltage-gated Na+ (6AGF) and K+ (1BL8) channels obtained by the molecular docking strategy. In this study, molecular dockings of Echium angustifolium constituents to channels were performed using Auto Dock 4.2 to investigate the binding mode of Echium angustifolium constituents to obtain information about interaction forces of Echium angustifolium constituents and voltage-gated channels. Echium angustifolium constituents and voltage-gated channels were kept as flexible molecules and were docked into seven forms of rigid ion channels to obtain their preferential binding site to Echium angustifolium constituents.
Chemical structures |
Chemical nature |
Name of ligand |
Na+ channel (6AGF) |
K+ channel (1bl8) |
|
ciguatoxin-1 |
P-CTX 1B |
-12.3 |
-8.6 |
||
pyrrolizidine alkaloids |
9-angeloylretronecine |
-5.5 |
-5.0 |
||
pyrrolizidine alkaloids |
7-angeloylretronecine |
-5.6 |
-5.0 |
||
pyrrolizidine alkaloids |
(7R, 8S)-petranine 3 |
-6.1 |
-5.0 |
||
pyrrolizidine alkaloids |
(7S, 8R)-petranine 1 |
-6.8 |
-5.0 |
||
pyrrolizidine alkaloids |
(7R, 8R)-petranine 4 |
-6.2 |
-5.1 |
||
pyrrolizidine alkaloids |
(7R, 8S)-petranine 2 |
-6.4 |
-4.9 |
||
Phenolic acid derivatives |
Gallic acid |
-6.0 |
-6.1 |
||
Phenolic acid derivatives |
Benzoic acid |
-5.8 |
-4.3 |
||
Phenolic acid derivatives |
Ellagic acid |
-7.8 |
-9.0 |
||
Phenolic acid derivatives |
Chlorogenic |
-7.0 |
-7.6 |
||
Phenolic acid derivatives |
Vanillic acid |
-6.0 |
-5.7 |
||
Phenolic acid derivatives |
Catechol |
-5.8 |
-5.3 |
||
Phenolic acid derivatives |
Salicylic acid |
-6.0 |
-5.6 |
||
Phenolic acid derivatives |
Ferulic acid |
-6.4 |
-6.1 |
||
Phenolic acid derivatives |
p- hydroxy- |
-5.5 |
-5.2 |
||
Phenolic acid derivatives |
Protocatechuic |
-5.7 |
-5.9 |
||
Phenolic acid derivatives |
P-Coumaric |
-6.0 |
-5.1 |
||
Phenolic acid derivatives |
Catechin |
-7.7 |
-6.0 |
||
Phenolic acid derivatives |
Rosmarinic acid |
-7.6 |
-5.6 |
||
Phenolic acid derivatives |
Dihydroxyphenyl lactic acid |
-8.9 |
-5.6 |
||
Flavonoid |
Naringin |
-8.2 |
-6.8 |
||
Flavonoid |
Rutin |
-8.4 |
-7.0 |
||
Flavonoid |
Hesperetin |
-8.5 |
-6.2 |
||
Flavonoid |
Hesperidin |
-7.3 |
-6.5 |
||
Flavonoid |
Naringenin |
-7.3 |
-5.8 |
||
Flavonoid |
Quercetin |
-7.6 |
-5.7 |
||
Flavonoid |
Apigenin |
-8.3 |
-5.7 |
||
Flavonoid |
Kaempferol-3-neohesperidoside |
-7.9 |
-8.4 |
||
pyrimidine-analog |
Uridine |
-6.2 |
-7.5 |
Table 2 Various energies in the binding process of Echium angustifolium constituents and voltage -gated Na+ (6AGF) and K+ (1BL8) channels obtained from molecular docking. The unit of all energies (ΔG) is kcal/mol.
The molecular docking results are shown in Table 2. The modeling studies show that there are van der Waals, hydrogen bonding and electrostatic interactions between Echium angustifolium constituents with voltage-gated channels. The contribution of van der Waals and hydrogen bonding interaction is much greater than that of the electrostatic interaction because the sum of van der Waals energy, hydrogen bonding energy and desolvation free energy is larger than the electrostatic energy, which is consistent with the literature.37,38 The Echium angustifolium constituents for example ellagic acid and voltage-gated K+ (1BL8) channel interactions are shown in Figure 2. Echium angustifolium constituents showed a significantly higher binding energy for ellagic acid (-9.0kcal/mol,) when compared to ciguatoxin-1 (-8.60kcal/mol) as mentioned in Table 2. Figure 2 shows six hydrogen bonds between ellagic acid and K+ voltage-gated sodium while ciguatoxin-1 has five hydrogen bonds with voltage -gated K+ channels. In addition, ellagic acid showed significant docking interaction with the voltage -gated K+ channels .binding site (threonine 75 and threonine 74) as shown in Figure 2. Similarly ciguatoxin-1 showed excellent docking interaction with the K+ channel. binding site (proline55 and glutamine 58). The interaction of ellagic acid with the K+ channel binding site is essential for effective reversing the blocking action of ciguatoxin-1. The voltage-gated potassium (Kv) channels family can be divided into several subfamilies on the basis of sequence function and similarity. Four of these subfamilies, Kv1 (Shaker), Kv2 (Shab), Kv3 (Shaw) and Kv4 (Shal), consist of pore-forming alpha subunits that related to dissimilar types of beta subunit. Dhruba et al.39,40 reported that there is a subtype-specific differences in the role for Kv1 channels and only Kv4 channels are involved in repolarizing the narrow action potential of mouse somatosensory cortex cells and this could explain the protective results obtained with KcsA. Therefore, some Echium angustifolium constituents may be considered as the effective agents of reversing the blocking action of ciguatoxin-1.
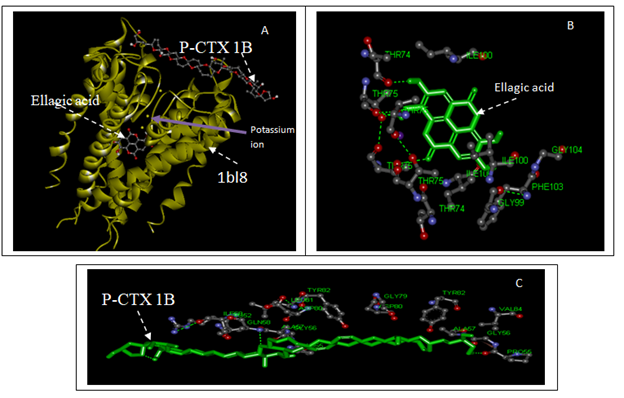
Figure 2(A) Shows the interaction model between ellagic acid and ciguatoxin with K+ channel (1bl8, KcsA (K+ channel of streptomyces)). (B) Shows the interaction model between ellagic acid with the K+ channel active site. (C) Shows the interaction model between ciguatoxin with the K+ channel. The hydrogen bonds are represented using green broken lines. The figure was obtained with the help of molecular visualization tool (discovery studio software 2.4).
Pyrrolizidine alkaloids are a group of naturally occurring alkaloids based on the structure of pyrrolizidine which are produced by plants as a protection mechanism against insect herbivores and some of them when used chronically are hepatotoxic. Pyrrolizidine alkaloids consist of a necine base esterified with a necic acid. The necine base characteristically includes pyrrolizidine, a bicyclic aliphatic hydrocarbon consisting of two fused five-membered rings with nitrogen at the bridgehead. Pyrrolizidine alkaloids are principally present as N-oxides (Table 2), which are good water-soluble and considered with esters as hydrogen bonding groups with amino acids of Na+ and K+ voltage gated channels.41,42 In this work, we assessed the capacity of Echium angustifolium aqueous extract to reverse the P-CTX-1B-induced toxicity in mice. The toxicity produced by ciguatoxin was inhibited by Echium angustifolium at dosages up to 411μl (1.29g/30ml). A comparison of the structures and activities of pyrrolizidine alkaloids, phenolic acid derivatives, flavonoids and pyrimidine-analog indicates that the carboxyl moiety of phenolic acid derivatives constitutes a significant substituent required for activity against ciguatoxin toxicity. In fact, these derivatives lacking this carboxyl functional group were not very potent in case of potassium voltage gated channels. In addition, flavonoids that contain phenols were a considerable substituent required for activity against ciguatoxin toxicity in case of sodium voltage gated channels. Additionally, other phenolic acid derivatives and flavonoid had positive binding energies. The phenolic compounds are still the most potent on both channels suggesting that the phenolic moieties were required for a significant positive activity against ciguatoxin toxicity. Furthermore, the similar activity of phenolic acid derivatives and flavonoids derivatives confirmed that the positive activity results from both the phenolic and carboxyl moiety. Lastly, the number of hydroxyl substitutions on the phenolic moieties was important. Indeed, the difference of activity between gallic acid, benzoic acid, ellagic acid, chlorogenic acid, vanillic acid, catechol, salicylic acid, ferulic acid, p-hydroxy-benzoic acid, protocatechuic acid, p-coumaric acid, catechin, rosmarinic acid, dihydroxyphenyl lactic acid, naringin, rutin, hesperetin and hesperidin showed that they needed at least two hydroxyl substitutions. Thus, a diphenol was required to obtain inhibition of the ciguatoxin toxicity. The comparison with all these derivatives indicates that the structure of phenol was significant for its biological activity. This specificity of action provides a basis for the improved acceptation of the wide utilization of Echium angustifolium aqueous extract, which contains pyrrolizidine alkaloids, phenolic acid derivatives, flavonoids and pyrimidine-analog, of future treatment of ciguatoxin toxicity.
In silico toxicity assessment of Echium angustifolium components
Echium angustifolium components were evaluated for drug-likeness and toxicity. Examination of Echium angustifolium components for drug-likeness was performed by computational prediction of ADME-Tox properties (adsorption, distribution, metabolism, excretion, and toxicity). All Echium angustifolium components were found to be non-carcinogenic and acceptable as drugs. In addition, all Echium angustifolium components were found to follow Lipinski Rule of five for drug likeness with molecular mass less than 500 daltons, no more than 5 hydrogen bond donors, no more than 10 hydrogen bond acceptors, with Log P scores not exceeding 5, and molar refractivity 40-130.26,43 The limitation of this study is the cytotoxicity assay, which still needs to be an essential part of evaluating the safety of Echium angustifolium constituents because it affords direct information at the cellular level which may be significant in assessing the true toxicity of Echium angustifolium constituents.
Ciguatera Fish Poisoning is seafood intoxication due to consumption of tropical coral reef fishes that have built up ciguatoxins in their tissues. Echium angustifolium aqueous extracts exhibit a positive activity in treating ciguatoxin toxicity. The results indicated that Echium angustifolium constituents form hydrogen bonds with active sites of Na+ and K+ channels and protect the mice from ciguatoxin toxicity. The positive activity in mice suggests a promising detoxifying action caused by cigua-intoxication. Furthermore, the obtained results confirm the potential of Echium angustifolium in the treatment of Ciguatera Fish Poisoning. Detailed clinical studies in this direction are needed to potentiate this claim in human beings.
The authors gratefully acknowledge the technical support and valuable suggestions obtained from Sir Ali Katane Sharef (Novelien Zone, Tripoli, Libya).
The authors declare there are no conflicts of interest.
None.

©2020 Sadawe, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.