MOJ
eISSN: 2471-139X


Review Article Volume 6 Issue 3
Professor of Anatomy and Embryology, Faculty of Medicine, Cairo University, Egypt
Correspondence: Heshmat SW Haroun, Professor of Anatomy and Embryology, Kasr Al Ainy Faculty of Medicine, Cairo University, Egypt
Received: January 06, 2019 | Published: June 26, 2019
Citation: Haroun HSW. Digito-palmar dermatoglyphics: variations and prediction of brain disorders. MOJ Anat & Physiol. 2019;6(3):103-106. DOI: 10.15406/mojap.2019.06.00254
Dermatoglyphics is the science describing the configuration and frequency of the epidermal ridges on the palms, soles and their digits. They are stable throughout life and remain unchanged unless the epidermis is destroyed. Dermatoglyphics are completely developed, from the ectoderm, by about the 18th intrauterine week and they stay unaltered in late prenatal and postnatal lives. Individual, ethnic, sex, age and laterality variations of dermatoglyphics do exist. Finger- and handprints are extensively used in criminology. Moreover, analysis of dermatoglyphics has been recently utilized in the diagnosis of some medical and genetic disorders. Dermatoglyphics could be used as predictors of some brain disorders like schizophrenia, autism, Alzheimer’s disease, congenital mental retardation, cerebral palsy, multiple sclerosis, dyslexia and hereditary sensorineural deafness.
Keywords: dermatoglyphics, ethnicity, sexual dimorphism, brain disorders
Digito-palmar dermatoglyphics is the scientific study of the morphology of the skin ridges on the palms and finger pads (Figure 1 & 2). They could be visualised by methods like vacuum metal deposition and cyanoacrylate fuming method,1 carbon paper and tape method ,2 scanner method3 and inked roll method.4 Sexual dimorphism and ethnic variations of digito-palmar dermatoglyphics have been extensively reported in the literature. Higher values of finger, palm and interdigital ridge counts were assessed in Bulgarian males who also had higher frequency values of Hy, Th/III and Th/IV patterns.5 Sex-related variations in epidermal ridge breadth (thickness or density) on whole palms of Caucasian Spanish population were detected: females showed significantly narrower ridges and more ridge density than similarly-aged males.6 In Chuvashian population of Russia, obvious sexual dimorphism of digito-palmar dermatoglyphics was also observed.7,8 On the contrary, no significant sex-differences were detected in the dermatoglyphic patterns of the five endogamous populations of West Bengal, India.9
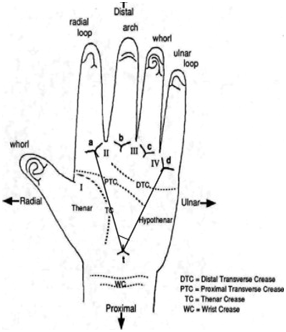
Figure 2 Digito-palmar dermatoglyphics: triradii, palmar creases and “atd” angle (from google images).
Digital and palmar dermatoglyphics of healthy individuals, of both sexes of two northern Sinai tribes, were found to be mostly alike and they resembled those of urban Egyptians and sometimes of neighbouring African tribes suggesting genetic admixture with north Africa.4 In Kosovo, quantitative analysis of dermatoglyphics, of both sexes, displayed small admixture between Albanian and Turkish populations; the Turks preserved their ethnic identity for centuries.10 In Quebec City, there was unilateral or bilateral absence of palmar c-triradius in 66.6% of individuals derived from a Canadian family line since the 17th century; this was attributed to an autosomal dominant trait of a heterozygous genotype.11 In extreme northwest of Nepal, a significant digito-palmar dermatoglyphic similarity was determined between the inhabitants of Buddhist-Tibetan villages and their Hindu-Caucasian neighbours.12 Furthermore, patterns of digital ridges were greatly similar among indigenous black Zimbabweans, Malawians, and some South Africans indicating a close anthropological relationship of these races.13
Age-related features of some palmar dermatoglyphics were used as a tool for determination of longevity of Ukrainians.14 Also, in Caucasians, 16-80 years old and of both sexes, lists of age-specific features of palmar dermatoglyphics were prepared for age-determination of unknown subjects.15
A relationship was established between digito-palmar dermatoglyphics and handedness of Bulgarians where some dermatoglyphics were more abundant in left palms than in right palms of right-handers particularly females.16 Laterality-differences in palmar dermatoglyphics were also assessed in Khatri males: higher termination of “line D” in right palms, frequently absent “line C” in left palms, decreased frequency of “Hy” patterns in right palms, increased frequency of the “Th/Ist” interdigital patterns in left palms, increased frequency of the “IInd” and “IIIrd” interdigital patterns in right palms, and of the “IVth” interdigital patterns in left palms.17
The range values of frequencies of different patterns of digito-palmar dermatoglyphics, in Limboo (18-60 years of both sexes) of Sikkim, India, were estimated as follows: loops (64.33% - 75.00%), whorls (21.33% - 31.00%) and arches (3.66% - 4.67%) lacking significant sex-differences.18 The range and mean values of frequencies of digito-palmar dermatoglyphics of Greeks were discovered to be nearly the same as those of other Caucasian populations.19 In endogamous Indians of West Bengal, higher mean values of “triradii” number, “adt” angle, and lower mean values of “td ridge” and “ab ridge” counts, and “ab ridge” breadth were observed in inbred individuals when compared to their non-inbred relatives.20 In Ndokwas people of Delta State of Nigeria, ulnar loops were more observed in digits III, IV and V whereas radial loops in digit II, with higher number of radial loops in females and higher number of whorls in males.21 On comparing digito-palmar dermatoglyphics in healthy South African Whites to those of healthy South African Negroes, Indians, and Coloreds (mixed racial origin), it was concluded that the Indians' dermatoglyphic profile was dominant to those of Whites and Negroes.22 Also in New Guinea, comparing digito-palmar dermatoglyphics of the Elema people to those of other peoples it was found that Elma had a high intensity index and frequency of whorls, and the highest frequency of ulnar type C line, complete simian creases and IV interdigital patterns.23 In Malawian subjects, arches were the most predominant digital pattern in both sexes, followed by radial loops in males and whorls in females; sexual dimorphism of these digital patterns was statistically non-significant.24 In another study on Kenyan and Tanzanian subjects, ulnar loops were the most predominant digital pattern and arches were the least with significant sex-differences exhibited in arches, ulnar loops and whorls, concluding that dermatoglyphics of Tanzanians were closer to those of Malawians than Kenyans.25
Digito-palmar dermatoglyphics particularly flexion creases are known to provide clues of early fetal development; altered creases indicate early intrauterine disturbances and they are predictive of hidden lesions in apparently normal infants.26
In schizophrenia
Dermatoglyphics and brain structure have a common developmental origin from the embryonic ectodermal germ layer, therefore dermatoglyphics are determinants of early disturbances in brain development in schizophrenia. Schizophrenia is an aberrant neurodevelopmental disorder marked by abnormalities in brain structure and dermatoglyphic traits. Directional asymmetry, ridge count (RC) of b-c and bilateral anterior hippocampal volume were found to be significantly lowered in schizophrenic patients than controls.27
Fluctuating asymmetry is a term applied to the differences between theoretically identical right- and left-sided structures; it is used for investigating developmental disorders. Digito-palmar dermatoglyphics in schizophrenics exhibited significantly higher levels of fluctuating asymmetry in the total finger ridge count (TFRC), fingerprint patterns, palmar “atd” angles and palmar ab-RC, than controls.28 Fluctuating asymmetry of the finger ridges and the volumes of the whole brain and left hippocampus are essential tools for identifying twins highly susceptibility to schizophrenia.29
Schizophrenic patients were observed to have increased number of genuine and defective patterns on the “Th” and “Hy” areas, and of the loops in the “IIIrd interdigital area.30 In another study, schizophrenics when compared to the controls, had a marked difference regarding the termination of the palmar C line, the number of ridges at the a-b interdigital area and the frequency of different palmar flexion creases.31
In autism
Analysis of dermatoglyphics could be used as an additional procedure in the diagnosis of autism. Boys with infantile autism, when compared with healthy controls, had a significantly higher bilateral arch count on fingertips particularly the 4th and 5th, lower loop count, lower TFRC on the ten fingertips and ab-RC as well as wider “atd” angle.32 Significant differences in fingers and palms RC were determined between the autistic patients and their healthy controls, and between the family members of autistic patients and their healthy controls. These differences were more pronounced between healthy female controls and female family members (autistic females and their healthy mothers and sisters). A genetic linkage between a recessive X-chromosome and the etiology of autism was proclaimed.33 Boys with autism spectrum disorders (ASD), when compared to typically developing boys, revealed a higher rate of discordance in their fingerprint patterns.34 In autistic boys from the Basque Country, digital dermatoglyphics significantly demonstrated a higher frequency of radial loops, a lower frequency of dicentric whorls and TFRC in addition to a lower frequency of a-b interdigital RC when compared to control boys. In autistic girls, palmar dermatoglyphics significantly had a lower frequency of radial loops in the “Hy” area, and higher values of "atd" angles when compared to control girls. In both autistic sexes, there was a higher frequency of aberrant palmar creases.35
In Alzheimer’s disease, mental retardation and cerebral palsy
Patients with Alzheimer's disease (AD), when compared to controls, showed a significantly increased frequency of ulnar loops on the fingertips, palmar Simian creases, “Hy” patterns, and large “Th” distal loops; together with a trend toward an increased frequency of palmar Sydney lines and radial loops on the 4th and 5th digits. Such dermatoglyphic changes, in AD patients, were greatly similar to those observed in Down's syndrome children and their parents, raising an assumption of a common genetic factor regulating meiotic non-disjunction during gametogenesis, early prenatal development of dermatoglyphics, and postnatal enhancement of neuronal senescence.36 On the contrary, another investigation failed to detect major differences in digito-palmar dermatoglyphics between patients with dementia of Alzheimer type (DAT) and healthy elderly controls; with absence of dermatoglyphic changes commonly observed in Down's syndrome (trisomy 21).37 Moreover, females with DAT, when compared to controls, displayed significantly increased number of palmar accessory triradii and complete Sydney creases; whereas DAT males had no similar dermatoglyphic changes; this study also failed to confirm similarity of these dermatoglyphic alterations to those of Down’s syndrome.38
In children with congenital mental retardation of unknown etiology, dermatoglyphic analysis is recommended as a routine tool of investigation.39 Quantitative analysis of digito-palmar dermatoglyphics in children suffering from cerebral palsy, revealed significantly lower total ridge count (TRC) of boys when compared to their fathers and significantly higher TRC of girls when compared to their mothers; hypothesizing a possible genetic predisposition particularly of a pronounced paternal role, for the occurrence of central nervous system damage.40
In multiple sclerosis, dyslexia and hereditary sensorineural deafness
There is a great evidence of a multi-genetical predisposition for multiple sclerosis (MS). A "MS Trait" triggers the reactivity of the immune system to ecological factors. The digito-palmar dermatoglyphic complex is also multi-genetically determined during early embryogenesis. MS patients, of both sexes, were found to show significant reduction of the digital and palmar ridge counts together with lower values of “atd” angles when compared to phenotypically healthy individuals of both sexes. Therefore, dermatoglyphic analysis is suggested as a possible discriminative tool between MS patients and phenotypically healthy individuals.41
There is a consensus that the causative factor for development of dyslexia most probably exerts its effect during early prenatal period when the palmar dermatoglyphics are formed. Dyslexics of both sexes, compared to healthy controls, were found to reveal dermatoglyphic changes, particularly on the left hand, like higher left a-b counts, wider bilateral “atd” angles, and greater pattern frequencies in left interdigital area IV.42
Boys and girls with severe recessive perceptive hearing impairment, and their fathers, showed digito-palmar dermatoglyphics different from those of healthy controls; a polygenetic inheritance of sensorineural hearing loss was proclaimed.43
Digito-palmar dermatoglyphics show pattern, count and frequency variations related to ethnicity, gender, age and handedness. A high frequency of dermatoglyphic changes or asymmetry is an indication of early prenatal insult. Analysis of the digito-palmar dermatoglyphics elaborates several parameters which could help as predictors of hidden brain disorders. This would help in the diagnosis and treatment of these disorders in examined individuals.
The author declares no conflict of interest.

©2019 Haroun. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.