MOJ
eISSN: 2471-139X


Mini Review Volume 1 Issue 5
1Department of Psychology, Central Michigan University, USA
2Department of Neuroscience Program, St. Mary's university of Michigan, USA
3Department of Physiology, University of Tennessee Health Science Center, USA
Correspondence: Panchanan Maiti, Department of Psychology, Central Michigan University, USA, Tel 19894973026, Fax 19894973119
Received: September 28, 2015 | Published: December 7, 2015
Citation: Maiti P, Manna J. Dietary curcumin: a potent natural polyphenol for neurodegenerative diseases therapy. MOJ Anat Physiol. 2015;1(5):127–132. DOI: 10.15406/mojap.2015.01.00026
Aggregation of misfolded amyloid proteins is a key factor for synaptic damage and impairment of neuronal communication in several neurodegenerative diseases. Since last few decayed several synthesized compounds, small molecules, drugs have been used to target against these misfolded proteins, but ultimately failed to prevent the misfolded protein aggregations and neurotoxicity effectively. Therefore, therapies for these diseases are elusive and under active investigation. As a potent anti-amyloid activity and its Pleotropic actions, recently curcumin has been used for treatment of several neurodegenerative diseases. It is the main ingredients of turmeric powder of the herb Curcuma longa. Its preferential binding properties with misfolded amyloid proteins attracted researchers to use as a potential therapy to prevent neurodegeneration. Importantly, curcumin is also a safe, inexpensive, easily available Polyphenol and it can cross blood brain barrier. Therefore, it is considered one of the promising natural Polyphenol for therapy of age related protein misfolding diseases, including several neurodegenerative disorders. In this mini review article we provided conceptual information about the multiple potentials of curcumin for prevention and/or treatment of neurodegenerative diseases.
Keywords: neurodegenerative diseases, amyloidosis, curcumin, neuroinflammation, anti-amyloid
Cc, cur-curcumin; Aβ, amyloid beta protein; NFTs, neuro fibrillary tangle; NF-κB, nuclear factor kappa beta; COX, cyclooxygenase; LOX, lipoxygenase; TNF, tumor necrosis factor; IL, interleukin; ROS, reactive oxygen species; RONS, reactive nitrogen species; HSP, heat shock proteins
Neurodegenerative diseases are age related, multifactorial, and complicated disorders of nervous system, which has no successful treatment or cure.1 Most of these diseases may onset with accumulation of misfolded proteins even more than decays before its clinical symptoms arise.1 Multifactors are involved to initiate these disease progressions, including neuro-inflammation, oxidative damage and accumulation of misfolded amyloid proteins.2‒4 These events may work either independently, or together and ultimately impairs neuronal communications by damaging the neurons, which results a long term cognitive and motor dysfunction.5 Therefore, to restore normal brain functions and to delay onset or progression of diseases, it is necessary to start therapy before disease start to progress.6,7 Although several efforts have been implicated to attenuated disease progression using anti-amyloid, anti-inflammatory agents, small molecules and drugs, but none of them are in satisfactory level. Recently, as a safe, inexpensive, anti-amyloid Polyphenol, curcumin (Cur) draw a special attention to the researchers to use as a promising drug of choice to combat against several complicated neurodegenerative diseases.7,8 It is and the principal yellow pigment (almost 77%) present in the turmeric root of Curcuma longa, and structurally diarylheptanoid in nature. Other two important component of turmeric powder are desmethoxycurcumin and bis-desmethoxycurcumin (Figure 1). Because of its anti-inflammatory properties, since more than five thousand years, curcumin has been used in Indian and Southeast Asian traditional Ayurveda medicine.9 However, last few years scientist discovered its promising anti-amyloidogenic properties and started using as therapy for neurodegenerative diseases.6,7 It can not only bind and inhibits the amyloid beta protein aggregation,10‒13 but also binds with Alfa synuclein,14 huntingtin,15 and prion proteins.16 Therefore, based on its potent anti-amyloid activity, Cur is a promising natural compound to combat against neurodegenerative diseases caused by protein misfolding (Figure 1). This mini review provided some basic information about curcumin therapy and their potential impact on neurodegenerative diseases.
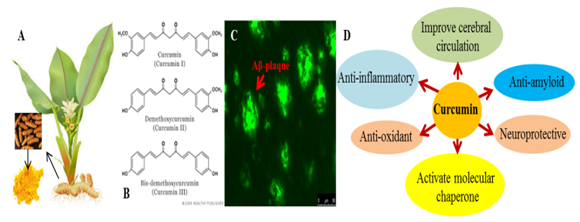
Figure 1 Curcumin- a natural Polyphenol, and an important health beneficiary component of the yellow powder of turmeric, which belongs to the roots of Curcuma longa, a herb from Zingiberaceae family.
Neurodegenerative diseases
Neurodegenerative diseases are the age related brain disorders, which causes worsening of many of our normal body activities, including our daily movement, balance or motor coordination, speech ability, respiratory and cardiovascular functions.3,17 In a simple word, this is a condition in which cells of the central nervous system are affected. Depending on the type of neurodegenerative disease and its affected area, the severity may vary. During the progression of these diseases, a massive demyelization takes place over time, lead to dysfunction and disabilities of many areas of brain, which normally control our normal body functions.18 Since last few decades, almost 30-40 different neurodegenerative diseases have discovered which are listed below.
Linked between protein misfolding and neurodegeneration
Proteopathies or protein misfolding diseases area the class of diseases, which causes impairment of neuronal communication. Many of the neurodegenerative diseases (but not all) involved protein misfolding and their abnormal accumulation in intracellular and extracellular spaces.8,19,20 For example, the most common age related neurodegenerative disease is Alzheimer’s disease (AD), which causes early memory deficits, followed by gradual decline of cognitive and intellectual functions or dementia.21 Aggregation of amyloid beta protein (Aβ) as senile plaque in extracellular spaces,22 and phosphorylated tau as neurofibrillary tangle (NFT) intracellularly23 are the cardinal features of AD. Similarly, accumulation of other amyloid proteins, such as α-synuclein, huntingtin and prion proteins are noted in Parkinson’s, Huntington and prion diseases respectively. Accumulation of all these misfolded proteins can cause impairment of synaptic communication, loss of synaptic integrity and impairment of daily brain function. These misfolded proteins can also impair cellular protein clearance pathways, including dysfunction of molecular chaperones, proteasome system and autophagy pathway.8 Therefore, restoring of these essential pathways would be a good strategy to remove these misfolded aggregates from the cells, and to preserve their normal function. Although, several small molecules, drugs, natural Polyphenol have been investigated to inhibit these misfolded proteins in these diseases, but none of them are in satisfactory level or not able to halt their aggregation completely, therefore therapy is elusive. Whereas, we found “curcumin” as a natural anti-amyloid Polyphenol, which have potential role to inhibit misfolded protein aggregation, and also restore protein clearance pathways,8,24 which are discuss further below.
Alzheimer's disease |
Parkinson's disease |
Huntington's disease |
Prion disease |
Creutzfeldt-Jakob disease |
Lewy body dementia |
HIV-associated dementia |
Cerebral palsy |
Pick's disease |
Corticobasal degeneration |
Progressive suprauclear palsy Amyotrophic lateral sclerosis |
Multiple sclerosis |
Ataxia telangiectasia |
Spino cerebellar ataxia Narcolepsy |
Spinal muscular atrophy |
Adrenal leukodystrophy |
Batten disease |
Bovine spongiform encephalopathy |
Familial fatal insomnia, |
Fronto temporal lobar degeneration |
Multiple system atrophy |
Primary alcoholism lateral sclerosis |
Schilder's disease |
Subacute combined degeneration of spinal cord |
Spielmeyer-Vogt-Sjogren-Batten disease |
Toxic encephalopathy |
Refsum's disease |
Sandhoff ‘s disease |
Alexander's disease |
Alper's disease |
Canavan disease |
Cockayne syndrome |
Kennedy's disease |
Krabbe's disease |
Neuroborreliosis |
Machado-Joseph disease |
Niemann Pick disease |
Pelizaeus-Merzbacher disease Steele-Richardson-Olszewski disease |
Tabes dorsalis |
Table 1 List of common neurodegenerative diseases
Diseases |
Proteins |
Pathology |
Affected area |
Complications |
Alzheimer’s |
Aβ, Tau |
Extracellular plaque, neurofibrillary tangle |
Hippocampus, amygdale, frontal, entorihnal cortex |
Memory loss, personality change, worried, depressed |
Parkinson's |
α- Synuclein |
Lewy body |
Substantia nigra, striatum, PFC |
Abnormal muscle movement, memory loss |
Huntington's |
Huntington |
Inclusion bodies in cytoplasm, nucleus |
striatum |
Uncontrolled movements, clumsiness, memory loss |
Prion |
Prion protein (PRPN) |
Prion plaques |
Whole CNS |
Memory loss, personality change, movement disorder |
Table 2 Misfolded protein aggregation involved in most common neurodegenerative diseases
|
Actions |
Mechanisms |
References |
|
Anti-amyloid properties |
Binds with Aβ and prevent its oligomerization & fibril formation |
|
|
Inhibition of Aβ production |
Inhibit activities of β-secretase (BACE), inhibiting amyloid precursor protein (APP) processing pathway |
|
|
Aβ clearance: |
Stimulate phagocytosis, thus decrease Aβ-plaques |
|
|
Inhibition of NFTs |
Bind with NFTs and inhibits tau phosphorylation (pTau) |
28 |
|
Inhibition of other amyloid |
Bind with α-synuclein in PD, huntingtin in HD and prion aggregates in prion disease |
|
|
Potent Antioxidant: |
Scavenges ROS/RONS, increase antioxidant levels, decrease lipid peroxidation, chelate toxic metals. |
|
|
Anti-inflammatory activity: |
Down regulate NF-κB, COX-2, 5-LOX, TNF, IL-1, IL-6. |
|
|
Regulate activity of molecular chaperones |
Restore levels of heat shock proteins (HSP90, 70, 60, 60, HSC70), protease system. |
8 |
|
Enhance NGF, BDNF, GDNF, neurogenesis & synaptogenesis |
Increase expression of BDNF, NGF, GDNF and can promote neurogenesis, synaptogenesis |
|
|
Improving cerebral circulation: |
Inhibits inflammation of brain vasculature leading to improvement of overall blood supply, reduce platelets adhesion in brain microvascular endothelial cell. |
32,33 |
Table 3 Pleotropic actions of curcumin to treat neurodegenerative diseases
Aggregation of misfolded amyloid proteins in the central nervous system is a leading cause of synaptic loss, neurodegeneration, and cognitive and behavioral impairment in several neurodegenerative diseases,1 which have no cure. Finding effective molecule, drug is vital to prevent or delay their further progression. As a potent anti-amyloid, anti-oxidant, anti-inflammatory Polyphenol, Cur has been widely investigated in the field of neurodegenerative diseases research.7,8 Though since last five thousand years Cur has been widely used for wound healing in traditional Ayurvedic medicine of India, and other South East Asian countries, but its Pleotropic actions, including anti-amyloid properties has been discovered last decay only.10,11
However, because of its potential impact to prevent and treat a wide spectrum of incurable and chronic diseases, nowadays Cur is globally accepted as one of the wonder drug for future.7 For example, recent research demonstrated that Cur can be used for Alzheimer’s, Parkinson’s, Huntington’s, prion’s diseases, multiple sclerosis, schizophrenia, depression, epilepsy, cerebral ischemia, and brain tumor.7,8,11,14‒16 It has been reported to reduce plaque burden and improve cognitive functions in mouse model of AD, and protected against Aβ-toxicity in vitro and in vivo.7,11,24,27 Despite strong evidence supporting the roles of Cur in inhibition of amyloid pathology in different brain diseases, its drug target is unclear. Further, how Cur can reduce these protein aggregates is poorly understood. However, we know that as a potent antioxidant, it can reduce oxidative stress, one of the leading causes of neuronal cell death noted in different brain disease.27 Similarly, neuro-inflammation plays a critical role in neurodegenerative disease pathogenesis, and as a potent anti-inflammatory agent, Cur can decline inflammation, thus prevent further pathogenesis.27 Furthermore, its preferential binding towards amyloid proteins and inhibition of their further aggregation could be the principal mechanism for an effective therapeutics to prevent neurodegeneration.10,11 Not only that, Cur also decreases tau protein aggregation; reduce soluble tau in human tau transgenic (HtauTg) mouse model.24 Another promising mechanism recently we observed is that it can regulate a common endogenous protein clearance pathway, such as the molecular chaperones or heat shock protein (HSP).8,24 Endogenous protein clearance pathway, such as HSPs have significant role in protein folding and maturation, and renaturation of misfolded proteins, thus play pivotal role to remove these aggregated proteins. This essential system is significantly down regulated in different brain diseases.8,24 Therefore, activation and or restoration of dysfunctional protein clearance pathways in different brain diseases by Cur would be a great strategy to remove the misfolded amyloid protein aggregates, and prevent or delay further neuronal damage in several neurodegenerative diseases.8
Protein misfolding and their accumulation inside or outside of neurons are the key pathological feature in several neurodegenerative diseases including Alzheimer’s, Parkinson’s Huntington’s and prion diseases. Several drugs, small molecules or natural compound have been investigated to inhibit these misfolded protein aggregations, but none of them are effective. Because of its strong amyloid binding capability, significant inhibitory effects of misfolded protein aggregation, and restoration of protein clearance pathways, Cur is considered one of the promising natural Polyphenol to combat against several neurodegenerative diseases. It is anticipated that the information provided through this mini review should help to researcher to get a conceptual detail about the Pleotropic actions of Cur for neurodegenerative diseases therapy
None.
Author declares that there is no conflict of interest.

©2015 Maiti, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.