MOJ
eISSN: 2471-139X


Research Article Volume 6 Issue 3
School of Veterinary Sciences, University of Chile, Santiago, Chile
Correspondence: Estefania Flores Pavez, Assistant Professor School of Veterinary Sciences, University of Chile, Santiago, Chile
Received: May 07, 2019 | Published: May 29, 2019
Citation: González CM, Cepeda R, Espinoza AV, et al. Anti angiogenic effect of thalidomide sulphate on canine transmissible venereal tumor. MOJ Anat & Physiol.2019;6(3):86-93. DOI: 10.15406/mojap.2019.06.00252
Among the new therapies to combat cancer the anti angiogenic drugs are gaining more and more importance. One of these compounds is thalidomide that had been removed from the market due to its teratogenic effects, but today is considered as a useful drug for the treatment of several illnesses in human beings. It has been used in neoplasias due to its inhibitory effects on angiogenesis, as well as its possible anti proliferative and pro apoptotic action on tumor cells.
In this study the effect of thalidomide was evaluated on the vascular bed of the canine venereal transmissible tumor (CTVT) of spontaneous presentation. For this purpose, thalidomide was administered to six dogs by a period of 14 days. Tumor biopsies were obtained at days 0, 7 and 14 after treatment and the vascular area was quantified by a computer assisted morphometric analysis of tissue sections immunostained for endothelial von Willebrand factor. The quantification of the tumor area affected by fibrosis was performed on tissue section stained with Masson’s trichrome stain.
The obtained results were statistically evaluated, obtaining a significant decrease (p<0,05) of the vascular area and a significant increase of the fibrosis degree in the analyzed tumors, after treatment.
Treatment with thalidomide induces regressive changes in the tumor as a consequence of its effects on the angiogenic activity, the increase of lymphocyte cell infiltration and the increment of the tumoral estroma.
Canine Venereal Transmissible Tumor (CTVT) is a round cell neoplasia of canids (Mohanty and Rajya, 1977) that is virtually the only tumor known to be transmitted by intact viable cells across the major histocompatibility complex barriers among adult immunocompetent allogenic dogs and other canines under natural conditions. This is apparently associated to low level of expression of MHC I and MHC II molecules on CTVT cells during progressive stage of tumor growth.1 The tumor is genetically distinct from its host and although is highly aneuploid, it has a remarkably stable genotype. Phylogenetic analyses indicate that CTVT most likely originated from a wolf or an East Asian breed of dog between 200 and 2500 years ago.2 CTVT has a worldwide distribution, and cytogenetic and immunologic studies demonstrated that tumors from different parts of the world are very similar.2 The precise cell type of the tumor is not quite clear. Immunophenotype support the hypothesis of a histiocytic origin for CTVT.3–6 A mutation of p53 gene at codon 316 has been reported in TVT tissues along with an insertion of a truncated LINE-1 element upstream of c-myc gene.7
Although spontaneously it only affects the dog, this tumor can be experimentally implanted in other canids like coyotes, wolves, jackals and red foxes.4
The natural transmission is through implantation of neoplastics cells during the coitus, or by licking affected genitals or other type of interactions between the affected animal and a susceptible guest.8 Thus, this tumor is described as a natural allograft of tumoral cells.9 These cells are implanted in epithelial surfaces, generally the genital ones, that have been damaged, most commonly in association to trauma during mating. Also biting, licking and scratching predisposes to cutaneous inoculation of tumoral cells,10 and explains extragenital CTVT cases in nasal and oral cavity.
The tumoral mass is friable and shows a red brilliant color due to its extensive vascularization, and often exudates a sero haemorrhagic fluid.9 These tumors bleed easily and as they grow they generally get both ulcerated and contaminated.11
The experimental evidence indicates that the growth and dissemination of a neoplasia depends on angiogenesis.12 The neoplasms express several proangiogenic factors. Although the FGF was the first one in being described, the VEGF is perhaps the most important. A reason for the widespread expression of VEGF is explained by its induction under ischaemic conditions. The neoplasms contain high levels of this factor in the borders of their hypoxic and necrotic tissues.13 Also, although the malignant cells are the main source of this factor the estromal cells and the vascular endothelium can also secrete it.
Immunohistochemical technic allow a better quantification of angiogenesis by using endothelial markers such as the vonWillenbrand factor (fvW), CD31 (PECAM-1),14 CD33 and CD34. Due to the reduced size of the new capillaries, it is difficult to detect them by light microscopy with Hematoxylin-Eosin stain.
One of the advantages of the antiangiogenic drugs is to be very specific, acting on molecules expressed on the surface of the activated endothelial cell, but not affecting the quiescente endothelial cells or other cell types. It would be the case of VEGF receptors, angiopoietins and some adhesion molecules such as integrins.
Thalidomide was synthesized in 1954 in east Germany as a substitute for barbiturates and it was prescribed as a sedative, tranquilizer and antiemetic in pregnant women.15 However, in 1961 it was attributed to this medication severe congenital abnormalities16 that included focomelia, dismelias, amelias, bony hipoplasticity and other congenital defects in ear pavilion, heart and internal organs. The teratogenic risk increased when the drug was administered between the third and eighth week after the conception.
In 1994, D'Amato and collaborators postulated that thalidomide had a direct effect on blood vessels growth. On the other hand, thalidomide has immunomodulatoy and antinflammatory properties. Thus, numerous studies with this drug have been developed in a wide range of immune and neoplastic illnesses.17 Undesirable effects are few and can be controlled by reducing the therapeutic dose.18
In Beagle dogs, doses of 43, 200 and 1000 mg/kg per day for a period up to 53 weeks did not show systemic toxicity or tumor formations. Some of the observed secondary effects were distension of the mammary gland excretory ducts and decrease of tyroxine levels.19
The mechanisms of action for thalidomide are rather complex. Besides the antiangiogenic effect, thalidomide increases the tumoral cell susceptibility to apoptosis, it stimulates the immune reponse, it reduces growth factors and it attenuates the potential of generating metastasis. This wide range of activities could be explained by its inhibitory effect on the activity of the nuclear kappa Factor (NF-k).20
The NF-k is activated in response to oncogenes, viral proteins, carcinogens, tumor promoters and inflammatory stimuli. Its activation on one hand controls the expression of genes that mediate the transformation, proliferation, invasion, angiogenesis, and metastasis, and on the other hand controls the apoptosis, immunity and hematopoyesis.21
More recent studies carried out in zebra fish; suggest that the antiangiogenic action induced by thalidomide is based on the balance between ceramide and esfingosin-1-phosphate (S1P), that regulates the angiogenesis through VEGF, Flk-1 and neuropilin-1 receptors. Ceramide has been described as a lipidic mediator that induces apoptosis. The ceramide is generated from the esfingomielin hydriolisis by means of the acid esfingomielasa (aSMase) or neuter esfingomielasa (nSMase). Thalidomide increases the activity of nSMase, therefore it is thought that the antiangiogenic action of thalidomide would be based on the depletion of VEGF receptors induced by ceramide.22
Also, it has been described that this drug would inhibit the Cyclooxygenase 2(COX-2) induction mediated by lipopolysacharids by means of the destabilization of its messenger RNA and consequently it would diminish the prostaglandin E2 biosynthesis, which regulates in a positive way the production of angiogenic factors.15
PGE2 is a potent inhibitor of fibroblast proliferation and collagen synthesis, thus in patients with pulmonary fibrosis, PGE2 levels are decreased.23 Several stimulatory mediators including transforming growth factor (TGF)-b1, platelet-derived growth factor (PDGF), insulin like growth factor-1, and thrombin as well as inhibitory mediators such as interferon, glucocorticoids, epidermal growth factor, and prostaglandin E (PGE)2 have been suggested to play a role in the pathogenesis of fibrosis.
In this study the canine Transmissible Venereal Tumor (CTVT) has been used as a biological model to assess the antiangiogenic activity of thalidomide, studying its effect on the endothelial area in tumor biopsies taken at different periods of time after treatment.
Animals
In this study six dogs sexually mature, without distinction of breed or sex, with genital TVT confirmed by cytology were considered. According to clinical aspects and tumor history the neoplasms were in progressive phase of tumor growth, this was also supported by their cytological and histopathological characteristics.
Treatment
The treatment was carried out with thalidomide (Talidomida LazarR, Lazar Laboratory, Argentine) in a daily dose of 8 mg/kg, administered orally, for seven days. Later on, the dose was diminishing in half, every other day, for another seven days. Three days after concluding the treatment with thalidomide, vincristine sulphate was applied at 0,03 mg/kg, i.v., once a week until total remission of the tumor was achieved.
Tumor sampling
Tumor smear and biopsies were obtained from all the animals at days 0, 7 and 14 during thalidomide treatment. The smears were air dried and then fixed in 100%methylic alcohol for 5 minutes. Then they were stained with Giemsa for 5 minutes and then mounted with cover lids to be examined under light microscopy.
Tumor biopsies were fixed in 10% formaline for 24 hours, then included in paraffin to obtain 4µm tissue sections, with a microtome, that were mounted on slides previously covered either with albumin for Hematoxylin-Eosin and Masson staining or xylane for immunohistochemistry.
Angiogenesis assessment
For the identification of the angiogenic activity blood vessels were immunostained using a primary polyclonal antibody against human von Willenbrand factor (DAKO®, Denmark), then a secondary biotinylated pig antibody against rabbit antibodies (DAKO®, Denmark), conjugated streptavidine/peroxidase, Streptavidin (Dako®, Denmark), and chromogen sustrate DAB (Dako®, Denmark) that gives a brown color to positive immunostaining.
The angiogenic activity was determined by quantification of the immunostained area. From each sample five optic fields with an augmentation of 200X, were obtained at random and digitized, using a scientific microscope (Nikon, Eclipse E600) connected to a video camera (Cool-Snap Pro, Media Cybernetics, USA) and to a computer with a morphometric analysis program (Image Pro-Plus, Media Cybernetics, USA). The software calculated the area a µm2 (Figure 1).
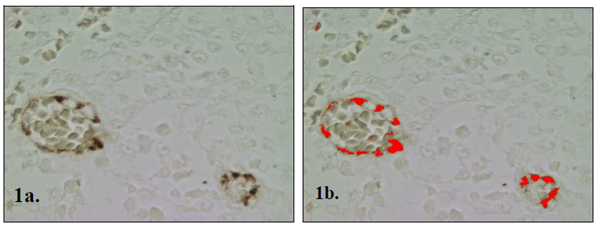
Figure 1 (A) Image of CTVT blood vessels immunostained with primary policlonal antibody against von Willenbrand factor ( brown color). (B) The red color indicates the area that has been recognized and measured in µm2 by the computer assisted morphometric analysis.
Fibrosis assessment
The histopathological analysis was carried out with Hematoxylin-Eosine (H-E) stained tissue sections, to study general morphologic characteristics of the tumor and with Masson stain to detect the increase of collagen type I associated to fibrosis. In order to calculate the amount of area occupied by collagen from each sample five optic fields with an augmentation of 200X, were obtained at random and digitized, using a scientific microscope (Nikon, Eclipse E600) connected to a video camera (Cool-Snap Pro, Media Cybernetics, USA) and to a computer with a morphometric analysis program (Image Pro-Plus, Media Cybernetics, USA). The software calculated the area a µm2 (Figure 2).
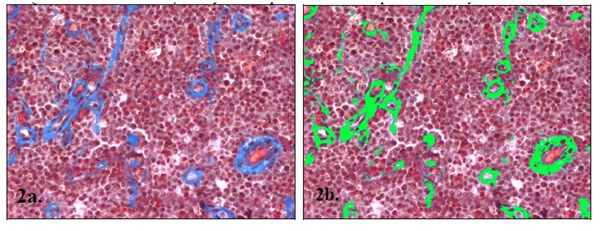
Figure 2 (A) CTVT tissue section with trichrome stain Masson where collagen fibers type I stain in blue. (B) The green color indicates the area that has been recognized and measured in µm2 by the computer assisted morphometric analysis.
Statistical analysis
The values obtained in the different days of treatment, for angiogenesis were analyzed by variance analysis in a factorial design 2x3" (ANDEVA), where the two factors to evaluate were:
The variables with a significant effect (p<0.05) were compared with the test of multiple ranges of Tukey.24
General tumor description
Tissue section of CTVT from day 0, before thalidomide treatment, revealed tumor features characteristics of the progressive phase of tumor growth. There was a prevalence of big, round or polyedric tumoral cells, with well defined nuclei and prominent nucleoli in a central or eccentric location. Mitotic figures were also frequently observed. CTVT cells were arranged in groups surrounded by few and thin collagen type I fibers (Figure 3). Small and occasional areas of perivascular infiltration by lymphocytes was observed.
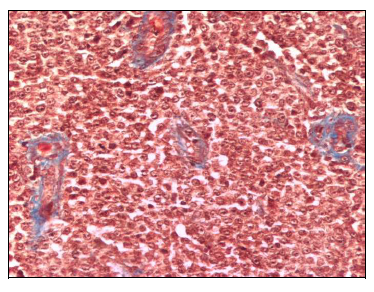
Figure 3 CTVT tissue section from day 0 previous to thalidomide treatment. Scarce and thin collagen type I fibers staining blue surrounding numerous tumoral cells. Masson Stain (200X).
In the samples from day 7 of thalidomide treatment some degenerative changes were observed in tumoral cells, such as cytoplasm vacuolation and nuclei swelling. Also an increase in lymphocyte infiltration was noticed. Collagen bundles also showed an increment in their number and thickness, giving the tumor a lobulated aspect (Figure 4).
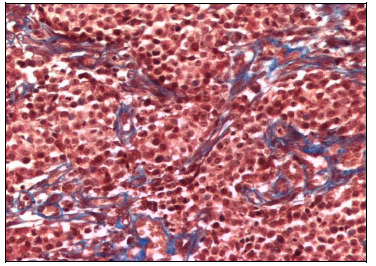
Figure 4 CTVT tissue section from day 7 of thalidomide treatment. More frequent and thicker collagen type I fibers staining blue surrounding tumoral cells with some degenerative changes. Masson Stain (200X).
At day 14 of treatment, with thalidomide, the changes were more noticeable especially the vacuolization of the cytoplasm, nuclear degenerative changes were more intense including intense swelling and picnosis. The typical histological arquitecture of this tumor was distorted by a more intense lymphocyte infiltration and fibrous tissue proliferation that dominated in some areas (Figure 5).
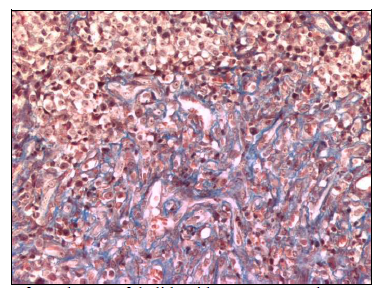
Figure 5 CTVT tissue section from day 14 of thalidomide treatment. A large area o fibrosis is observed with some infiltrative lymphocytes and vacuolar degenerative changes in remaining tumoral cells Masson Stain (200X).
Most of the CTVT cases studied showed clear morphologic changes during treatment with thalidomide except for case 6.
A prominent angiogenic activity was noticed at day 0 before treatment (Figure 6) with numerous capillaries and frequent branching from small blood vessels. After beginning treatment with thalidomide the presence of capillaries and endothelial buds diminished noticeably, remaining mostly blood vessels of bigger caliber, that were frequently collapsed and surrounded by fibrous tissue (Figure 7 & 8).
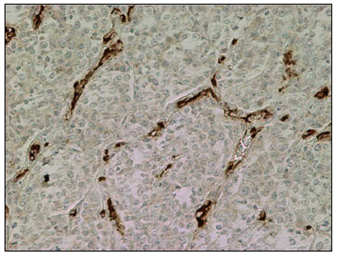
Figure 6 CTVT tissue section from day 0 previous to thalidomide treatment. Numerous and branching capillaries surround groups of tumoral cells. Immunohistochemical staining with Ab against von Willenbrand factor. (200X).
Collagen area
Previous to treatment with thalidomide (day 0) the average collagen area corresponded to 3.468,22 µm2 (Figure 9). Case 6 presented the biggest collagen area reaching 5.444,58 µm2 (Figure 10). The average collagen area increased at day 7 of treatment with 6.626,24 µm2.
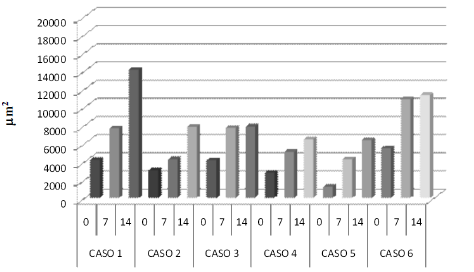
Figure 10 Average collage area (µm2) of each case of genital TVT at 0, 7, 14 days with thalidomide treatment.
Figure 9, existing a significant difference (p<0,05) with day 0. The analysis of individual patients (Figure 10) showed significant difference (p<0,05) among days 0 and 7, for cases 3, 4 and 5. The other three cases although showed a tendency to a bigger collagen area they had no significant differences (p>0,05).
At day 14 the average area was 8.987,83 µm2, being significantly different (p<0,05) in comparison to average areas of days 0 and 7 of treatment (Figure 9). Considering individual cases separately the difference was significant (p<0,05) in five of the six cases in comparison to day 0. Only case 6 did not present significant differences when comparing the three days (Figure 10). Only case 2 showed a significant difference (p<0,05) when comparing day 7 with day 14 of thalidomide treatment (Figure 10).
Vascular area
The average vascular area for the six CTVT cases studied at day 0, before treatment with thalidomide was 1.513,21 µm2 (Figure 11).
The average vascular for day 7 of thalidomide treatment corresponded to 730,76 µm2 (Figure 11), showing a significant difference (p<0,05) in comparison to day 0.
In every individual case a decrease of the vascular area was observed among day 0 and 7 of the treatment, being statistically significant (p<0,05) in five of the six cases (Figure 12). At day 14, the average vascular area was 365,40 µm2 (Figure 11), being statistically different (p<0,05) in relation to the average vascular area of day 7 and day 0. However when observing the individual results, only two of them presented significant differences (p<0,05) among day 7 and 14 of treatment with thalidomide (Figure 12).
The histopathological analysis of CTVT at day 0, before starting thalidomide treatment, revealed tumor characteristics corresponding to the progressive phase of growth described for CTVT.25 The only exception was case 6, which showed characteristics of an early regressive phase of growth, since degenerative changes were observed in CTVT cells and there were abundant dense collagen fibers.
The type of histological changes observed during treatment with thalidomide such as degenerative changes of tumor cells, fibrosis and lymphocyte infiltration are similar to those changes described for CTVT regressive phase of growth, after chemotherapy with vincristine sulphate.25 The increase in the tumor infiltrative population of lymphocytes, after the treatment can be partly associated to the effect observed by Teo26 who points out to that thalidomide acts as a co stimulator of T lymphocytes, inducing proliferation, cytokine production and increasing the cytotoxic activity.27
The results obtained in this study demonstrated a decrease of the endothelial area during the treatment with thalidomide, which is compatible with the antiangiogenic effect of this drug described for the first time in development of rabbit cornea28 and in multiple myeloma in human patients.29 The results described for different neoplasias are varied, thus, antitumoral activity has been described in Sarcoma of Kapossi, in the hormone-refractory prostate cancer17 and in a case of hepatic hemangioendothelioma.30 While in cases of colorectal cancer, carcinoma of renal cells, breast cancer, gliomas of high degree, melanoma and squamous cell cancer of head and neck, show any or very little antitumoral activity when it is used alone, but its effects improve when is administered in combination with other chemotherapy drugs.20,31
Angiogenesis depends on several factors, i) that the activated endothelial cells proliferate to allow the growth of the vessel, ii) the endothelial cells should secrete metalloproteinases required for dismembering the extracellular matrix that surrounds them, iii) they should be able to be move.
The decrease of endothelial area observed during the last days of treatment affected mainly newly formed capillaries and endothelial bud formation, resulting in a relative or proportional increase of blood vessels of bigger caliber, especially of the arteriolar type. This effect would largely be related to the ability of thalidomide of inhibiting the production of pro-angiogenic factors, such as VEGF, which is fundamental for the development of new blood vessels.20
Komorowshi et al.32 determined in vitro that thalidomide inhibited the liberation of VEGF, it diminished the formation of capillaries and the migration of endothelial cells. Also, the adhesion of these cells to collagen was increased, which is an indication that the blood vessel is stabilized and not in the angiogenesis process. These results help to explain the increase in the proportion of blood vessels of bigger caliber observed in this work.
Vincristine is a antineoplastic chemotherapeutic agent that binds to tubulin dimers, inhibiting assembly of microtubule structures. Disruption of the microtubules arrests mitosis in metaphase therefore affects all rapidly dividing cell types specially cancer cells.33 CTVT treated with vincristine sulphate, did not show a significant decrease of vascularization,25 this probably is due to the fact that this drug primarily and very quickly destroys CTVT cells and although it may affect other proliferating cells such as angiogenic endothelial cells, in this case it may not be possible to demonstrate a decrease in endothelial area in a tumoral mass where the neoplastic cell component is also decreasing and probably faster.
Although the treatment with thalidomide did not eliminate the established blood vessels, in this study had a positive effect in blocking angiogenesis avoiding the formation of new blood vessels. This type of vessels are also different as they are leaky presenting fenestrations34 that allows not only a nutritional support for a tumoral progressive growth but also promotes dissemination to the rest of the organism.
Also thalidomide blocks NF-kb35,36 which is one of the transcription factors that control the promoters of MMP, avoiding the degradation of the extracellular matrix which is fundamental both for angiogenesis and for tumor growth and invasion.
The fibrosis observed in this study, increased as the thalidomide treatment progressed. This does not agree with other authors who describe thalidomide like an effective antifibrotic in certain affections, as myocardium infarct, hepatic necro inflammation and cirrhosis.37,38 Apparently thalidomide would have this effect by decreasing the expression of TGF - 1 RNAm which is an important factor in remodeling the extracellular matrix and in fibrosis development.37 It is necessary to consider that they describe this antifibrotic effect of thalidomide in situations where fibrosis is associated to necrotic and inflammatory events, whereas the fibrosis observed in this thalidomide treated CTVT is apparently associated to hypoxia. Also, these authors worked with animal species different to dogs and it has been described that this drug has effects that are specific according to species.39
Spontaneous CTVT served successfully as an in vivo model to study the antiangiogenic effect of thalidomide. The effect can be more readily demonstrated if CTVT is in a progressive phase of tumoral growth as at this stage there is an important angiogenic activity. Later, when the tumor is in any degree of regressive phase of tumoral growth with a well established vascularization, as it occurred with case 6 of this study, it is difficult to have a significant effect.
In this study, under a rather short treatment schedule, it was possible to demonstrate an antiangiogenic effect of thalidomide in CTVT through the quantification of vascular area by a computer assisted morphometric analysis. Unlike the treatment with vincristine sulphate,25 clinically the tumoral mass underwent some reduction in size, but not always was significant (data not shown). It would have been necessary to have a more prolonged treatment with thalidomide to reach a more significant clinical effect. Also, the combination with other adjuvant drugs may potentiate the antineoplastic role.40
Clearly, the major challenge in treating cancer is not eradicating the primary tumor, which can be treated with radiation or surgery, but avoiding metastases which strongly depends on angiogenesis. If a tumor has not metastasized, or spread to other areas, and has been effectively treated with anti angiogenesis agents, metastasis is much less likely to occur because fewer blood vessels are available to spread cancer cells from the tumor.
On the other hand, very frequently, tiny, microscopic metastases in areas of the body far away from the primary tumor will remain inactive for years and begin to grow only after the primary tumor is removed. This happens because the primary tumor has been releasing angiogenesis inhibitors into the bloodstream, and when these inhibitors are gone, the microscopic tumors begin to grow. Cancer researchers hope that by using these angiogenic inhibitors as a maintenance therapy they can prevent these microscopic metastases from growing after the primary tumor is removed.
None.

©2019 González, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.