MOJ
eISSN: 2471-139X


Research Article Volume 5 Issue 3
1Cardiac Anatomy Research Laboratory, Instituto de Neurobiologia, Argentina
2Heart Laboratory, JJ Naon Morphologic Institute, Argentina
Correspondence: Almendras Isadora, Heart Laboratory, JJ Naon Morphologic Institute, Universidad de Buenos Aires, Paraguay 2155 (C1121ABG), Buenos Aires, Argentina
Received: January 08, 2018 | Published: June 14, 2018
Citation: Abuin G, Nicolás P, Isadora A, et al. Anatomy (real anatomy) of the aortic valve replacement: when we can actually see it. MOJ Anat & Physiol. 2018;5(3):217–219. DOI: 10.15406/mojap.2018.05.00196
Cardiac surgeons perform a number of procedures in the aortic outflow tract, the so named “aortic annulus” and the surrounding structures. This region has many structures related to the conduction system, like the atrioventricular node, the His bundle and its branches, plus the vascular supply of these elements.
There are several “dogmas” regarding valve replacement procedures that preclude any stitch from going “near”, “under”, “beneath”, etc. some specific zones; most of the time this dogmas are followed without reaching comprehensive understanding of the milimetric nature of the “play-field” when performing an aortic or mitral procedure.
The detailed anatomy of the subaortic outflow tract is clearly illustrated by the pictures accompanying this paper. There are not “schematic drawings” in this paper. All of the pictures are photographs of human hearts where dissection of the conduction system and its blood supply was performed. Some of the hearts were subjected to true cardiac procedures in vivo, and the dissection of the His bundle was performed postmortem.
This study reveals the precise location of the subaortic structures evidencing the real manoeuvre margin that the surgeon has in stented-valve, nostented-valve, Bentall and homografts procedures.
Ross procedure,1 aortic valve sparing operations, homograft placement, Bentall procedures and even standard aortic valve replacement can damage the atrioventricular bundle, the membranous septum or the blood supply of the atrioventricular node.2 For instance: the first “dogma” instructed to a resident in aortic valve replacement is: “Do not put any stitches under the annulus: if you do this, the patient can develop atrioventricular block”. The fact of the matter is that there is no aortic annulus, and when a stentless valve has to be placed in the “annulus” the sutures HAVE to be placed in an annular fashion. So, what is the truth? Where are the atrioventricular node and the His Bundle?
Performing the extraction of the pulmonary graft in the Ross procedure3,4 there is risk of damaging the first septal artery, resulting in bleeding and rhythm disturbances explained by the trajectory the vessel has. Accordingly, another dogma is: “When the autograft is extracted, some blood must be injected in the left coronary ostium and if there is no bleeding in the area of the excision, the haemostasis is secured”. However, there is another vessel shown in several pictures of this article supplying the atrioventricular bundle, the His bundle and the superior portion of the ventricular septum, the traditional left sided septal artery territory.5 This vessel is a branch of the RIGHT coronary artery and not of the anterior descending artery. The right superior septal artery (name of the referred vessel) also supplies the atrioventricular node and describes a course closely related to the right coronary ostium, as will be evidenced in the pictures of the present work. This artery will cut or ligated during the construction of the coronary buttoms for a Bentall procedure.
The arteria anastomotica auricularis magna or Kugel`s artery6 can be damaged during a Bentall or David procedure. The stitches of a subcoronary implantation of an homograft or a stentless valve can directly ligate the His bundle. In the pictures accompanying this article the real anatomy of the surrounding aortic structures will be exposed. No schematic drawings are used for this purpose, because it is the evidence the anatomy provides that should to convince the surgeon.
30 hearts were used for this study: all specimens correspond to human hearts injected with Microfill® or latex Carolina® in a retrograde fashion as described in previously published works.
The hearts were obtained from autopsy specimens; in some of the specimens cardiovascular surgical procedures were performed including Bentall procedures, standard valve procedures and subcoronary implantation of a homograft. In these specimens the His Bundle was dissected without excise of the aortic prosthesis.
Standard aortic valve replacement: In Figure 1A, the left atrium has been extracted, the aortic wall and the mitral valve are clearly distinguishable. The intervalvular trigone (3 in Figure 1A) is the uppermost demonstration that the aortic annulus does not exist; also in this Figure, the asterisks point the mitral commissures. When performing an aortic valve replacement, this is area constitutes a “secure” place to take good bites of tissue. This is the common site of location of the aortic abscesses causing “dislodgment” of the mitral valve. As it can be clearly seen, there is a space to perform a secure anchoring of the mitral valve to the aortic “annulus” if this structure is compromised by infection.7 The line between the anterior mitral valve and the intervalvular trigone is the site of insertion of the anterior wall of the left atrium. In Figure 1B, a simulation of a standard aortotomy for an aortic valve replacement can be appreciated as seen by the surgeon during the procedure, without the aortic walls.
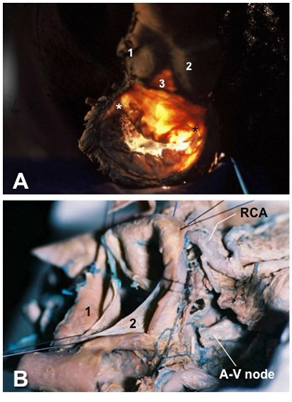
Figure 1 Standard aortic valve replacement. (A) Superior view of the mitro-aortic region of the heart; the intervalvular trigone can be appreciated (3) between the aortic and the mitral valve clearly demonstrating that the aortic annulus does not exist. Asterisks (*) are positioned in the anterior and posterior mitral commissures. (B)Right-superior view of the aortic valve showing the aortic leaflets and sinuses; Note that the atrioventricular node (A-V node) was dissected in the right side of the heart; a small artery supplying the atrioventricular node is also exposed, this vessel could be damaged during procedures such as aortotomy or enlargement of the annulus.
Abbreviations: RCA, right coronary artery; 1, left coronary artery and sinus; 2, non coronary sinus; 3, intervalvular trigone.
The membranous septum and stitches
In a competitive residency program, the anatomy of the aortic annulus is reviewed in every aortic procedure. Considering that, with no doubt, may the 1% or less of the ACTUAL cardiac surgeons ever dissected a human heart; the only source of “hands-on” anatomical learning experience for the treatment of the aortic annulus is carried within the huge limitations of the surgical field.
In Figure 2, the photographs were taken in a non attitudinal fashion, that´s to say, in a non standard anatomic nor surgical position, however, the landmarks are so clearly visible that the orientation in the operating room should be attained without major difficulties.
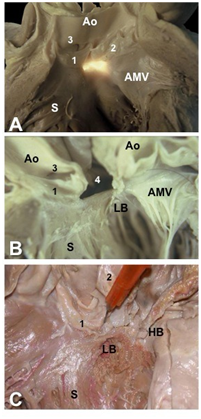
Figure 2 Membranous septum and the stitches. Left view of the mitro-aortic region. (A) Transillumination of the membranous septum showing its relationship with the aortic leaflets and the anterior mitral leaflet (AMV). (B) In this heart the membranous septum was excided (4); note the distance between the left bundle of His (LB) and the so-called aortic annulus. (C) Detail of the relationship between the His bundle (HB) and the non-coronary cusp (2); the pin is located in the membranous septum.
Abbreviations: Ao, aorta; S, ventricular septum; 1, right coronary sinus and leaflet; 2, non coronary sinus and leaflet; 3, right coronary ostium; 4, excised membranous septum.
The left bundle path is located at a prudent distance of the so-called aortic annulus (Figure 2), a needle anchored at the level of the nadir of the sinuses will never reach the bundle. Thus, if the stentless valve is to be implanted, attention should be paid in order to take bites of sutures under an imaginary plane crossing the nadirs of the three coronary sinuses.
The bentall procedure
The so-called Bentall procedure and its variations constitute surgical procedures that have successfully passed the proof of time. They are recognised as a trustworthy alternative for the treatment of aneurysms of the ascending aorta with no possibility of aortic valve preservation. It is well known that patients undergoing this surgery might be inclined to develop supraventricular arrhythmias, most of them transient and benign in origin and recurrence. The genesis of this topic has many explanations, but the morphological explanation is not well known.
Several arterial branches supplying the atrioventricular node and the bundle of His could be damaged during the Bentall procedure. The superior septal artery, branch of the right coronary artery, gives out a number of small vessels to the atrioventricular node, the His bundle and the right and left bundle branch. This vessel, well known up to the times of Kugel and Gross, has in fact no clear statistic demonstration of its role regarding the blood supply of the upper ventricular septum and the conduction system.8–11
In the Bentall procedure the right coronary ostium is implanted in the graft (1 in Figure 3); a small artery (2 in Figure 3), the right superior septal artery, traverses the area towards the atrioventricular node, making a star-complex anastomotic network with the atrioventricular node artery. This vessel could be clearly damaged during this kind of operation, and its knowledge should avoid this circumstance.

Figure 3 Simulation of Bentall procedure. The heart is observed from the right side, the right ventricle has been opened. The tricuspid valve, the atrioventricular node (A-V-node) and His bundle (HB) are evident.
Abbreviations: Ao, aorta; 1, right coronary ostium implanted in the graft; 2, right superior septal artery.
The aortic valve surgery is a standardized definite procedure; like Bentall procedure and his “brothers and sisters” Yacoub, David and other types of aortic wall reconstruction.
The variability of the anatomy of the blood supply of the heart conduction system does not affect the outcome of the surgical procedures, however by performing the procedure knowingly and acknowledging the potential risks the cardiac surgeon can manage the aortic zone with elegance, reducing the damage inflicted to the heart conduction system or its blood supply.12,13
For instance when performing the Yacoub procedure, the non coronary sinus should be resected a few millimetres away from its apparent attachment to the left atrium (the aorto-mitral continuity is hidden by the anterior wall of the left atrium in this area). By doing so, the Kugel´s artery should not be damaged.
In a mitral valve replacement14–17 it is strongly recommended to perform the procedure via lateral approach, because the biatrial approach at the level of the anterior wall of the right and left atrium destroys the vascular architecture of the cavities. If the surgeon is forced to access the left atrium in this last way, it is strongly recommended for the incision to be placed far away from the aortic insertion of the left atrium. In this way, Kugel´s artery is protected.
In the Bentall procedure, care must be taken when approaching the inferior aspect of the right coronary artery, because in this place, the superior septal artery frequently merges. This vessel, if injured, may cause two problems: haemostasis problems or cardiac block, often transient, caused by ischemia of the atrioventricular node.
As stated before, this article aims to give an insight into the anatomical details surrounding standard cardiovascular surgical procedures. Therefore, providing a background for a comprehensive approach of the physiological and functional repercussions arising from the mentioned procedures and their respective variations.
While acquiring familiarity with the anatomy of the region, surgeons are invited to perform chirurgical procedures in a precise and accurate manner, since reaching a better understanding of the area will positively influence on judgement at decision-making scenarios.
None
The author declares no conflict of interest.

©2018 Abuin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.