MOJ
eISSN: 2471-139X


Research Article Volume 7 Issue 6
1Department of Medicine, Tiradentes University, Brazil
2Department of Morphology, Federal University of Sergipe, Brazil
Correspondence: Francisco P. Reis, Department of Medicine, Tiradentes University, Brazil, SQS 214 Bloco H, Ap. 104, Brasilia, Distrito Federal, 70.293-080, Brazil, Tel +55-79-9-9981-6865
Received: October 21, 2020 | Published: November 10, 2020
Citation: Almeida RR, Matos CC, Aragão JA, et al. Anatomical and morphometric study of tracheobronchial angles in human fetuses. MOJ Anat Physiol. 2020;7(6):162-167. DOI: 10.15406/mojap.2020.07.00308
Purpose: Tracheobronchial angles (TBA) in human fetuses have been increasingly relevant in perinatal medicine to determine normal and pathological criteria adapted to anatomical particularities of the fetal tracheobronchial tree. The present study aimed to establish, in human fetuses, in situ, through morphometry, the dimensions and quantifications of the tracheal angles, relating them with gestational age and gender.
Methods: An observational and cross-sectional analytical study was conducted at the Anatomy and Tiradentes University’s Forensic Investigation Research Center. For research, the fetuses were selected taking into account the gestational age. Thus, 41 fetuses of both sexes, aged between 20 and 38 weeks, were selected, classified and maintained in 10% formaldehyde solution. The tracheas and the main right and left bronchi were evaluated in situ, and images were obtained, from which digital analyzes were performed.
Results: The tracheal bifurcation angle offers many discrepancies between the reported values in relation to the dependent sex and age differences. There were no significant differences regarding gender. There were significant differences for total TBA in terms of age. Regardless of sex and age, the right bronchial angle was smaller than the left. Angles increase from 20 to 32 weeks and decrease from 33 to 38 weeks.
Conclusion: Tracheobronchial angles, regardless of age and sex, do not correlate with the number of cartilages, nor with the trachea total length, distal and proximal length of the trachea. The present findings may represent an important knowledge to endotracheal intubation, procedures in the tracheobronchial tree, pulmonary physiology studies and anthropometry pure.
Keywords: tracheobronchial angles, right bronchial angle, left bronchial angle, morphometry, human fetuses
The word “morphometry” comes from the Greek radical - morphé, which means “form”, and from the Greek radical - metrikós, or from Latin - metricu, which means “the act of measuring or the process of establishing dimensions.” Although this term has wide application in science, its meaning in biomedicine would ultimately "the activity of measuring anatomical structures." The objective of using the morphometric method is becoming more objective and accurate the results obtained in research in addition to relate the different anatomical structures to their function.1 Pardine2,3 he noted that the morphometry may also be used to determine the dimensions and quantification of biological structures, contributing in this way to information on anatomical and pathological conditions.
The trachea is a tube formed by cartilage (cartilage rings) and a fibromuscular membrane, lined internally with mucosa. Its anterolateral part consists of incomplete cartilaginous rings and the posterior aspect by a flat muscular wall. The larynx extends from the sixth cervical vertebra level to the upper edge of the fifth thoracic vertebra, where it divides the so-called two main bronchi (lung) right and left. To Standring et al.4 the trachea, would be located approximately in a sagittal plane, but its point of bifurcation, to form the two main bronchi would be generally located slightly to the right.4
The fetal period begins at the ninth week and lasts until the birth. It is characterized by tissue and organ maturation and rapid body growth. In general, the fetus was born between 26 to 28 weeks have a 90% chance of survival.5 A more accurate knowledge of normal growth and development of the fetal airway to determine normal and pathological criteria has led to significant advances in perinatal medicine, in particular to the anatomical particularities of the fetal tracheobronchial tree.6
There are many studies on the development of the trachea and lungs. However, most it refers to a very early stage of pregnancy or the final stage of alveolar growth and the formation of surfactant. Most studies show that little attention has been given to the transition period between 3-8months of pregnancy. It has been considered that during this period, the main structural elements of the trachea are developed and where morphological changes may occur. This relatively long period of development appears to be significantly important for the use of endoscopic techniques for the early diagnosis of bronchial diseases.7,8
The rapid progress of perinatal medicine has allowed many different interventions in the tracheobronchial airways of fetal and newborn infants, as airway surgery, endotracheal intubation and bronchoscopy tracheostomy.6,9 In this way can be detached clinical and surgical point of view, the importance of knowledge of tracheal anatomy. An important example is the knowledge of the morphology of the trachea as helpful to select the correct size of the endotracheal tube.10 Although it is a very important procedure, the selection of an endotracheal tube of appropriate size for use in premature infants, based on clinical reports and industry recommendations, it may be accompanied by numerous limitations.10
Attempts fetal intubation or airway interventions in general are not performed before 24 weeks of gestational age.6 This seems to show that still look incomplete or even distant full knowledge of the quantitative anatomy of the trachea. However, morphometric data of fetal trachea has been relevant in prenatal surgery involving the tracheobronchial tree.6
Another important contribution of anatomical knowledge of the trachea and its dimensions in fetuses and neonates with regard to prenatal diagnosis and monitoring of tracheal defects and abnormalities such as agenesis, atresia, tracheal stenosis, short trachea, bronchiectasis primary tracheomalacia, tracheal diverticulum, traqueomegalia, pneumonia, bronchial obstructions, foreign bodies, mediastinal masses.6,9
At the sternal angle level, the trachea divides into its two main bronchi: right and left. Each major bronchus is then segregated again as it descends into the lungs, forming lobar and segmental bronchi.9 Tracheal bifurcation angle or tracheobronchial angle refers to the interbronchial angle, measured between the central axes of the right and left main bronchi, and the subcarinal angle, measured along the lower edges of the two main bronchi (Figure 1).11
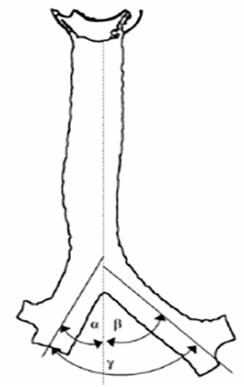
Figure 1 Right (α), left (β) and total (γ) tracheobronchial angle.11
Different studies on the angle of tracheal bifurcation in general have involved adults, children or both together.1–6 For calculations of the values of this angle, some researchers used plain chest radiographs or helical computed tomography (CT), while others used corpses. Almost all of these studies showed significant variations and discrepancies in the measurements of: tracheobronchial angle; tracheal length; right and left main bronchial angles; thoracic, tracheal and bronchial diagonal diameters.1–8,12,13
The present data from the anatomical and radiological literature have usually been referred mainly to tracheal bifurcation angle measurements in newborns, children and adults.14 Meanwhile, most radiographic studies have been severely limited due to lack of standardization regarding respiratory phase, type of projection, and degree of rotation or posture. The angle of the tracheal bifurcation tends to decrease by up to 9° between inspiration and expiration and the vertical deviation of the left main bronchus is considerably greater than that of the right main bronchus, as a consequence the left bronchial angle is much larger than the right.14
There are descriptions of the dimensions of the trachea and tracheobronchial angle in humans: Caucasian, western countries and for some regions of India.15 These authors also reported differences in values between the tracheobronchial angles in children compared to adults.
Knowledge of the anatomy, in particular, of tracheobronchial angles is of great relevance to the location of foreign bodies introduced into the tracheobronchial tree. It may also contribute indirectly to: the diagnosis of heart disease, mediastinal abnormalities, surgical resection of segments or lobes in some lung diseases, and help in choosing the correct size for a bronchoscope or certain tubes in more invasive procedures.9,11,16 Tracheobronchial angles in human fetuses have been increasingly relevant in perinatal medicine to determine normal and pathological criteria adapted to the anatomical particularities of the fetal tracheobronchial tree.14
The tracheal angle was also correlated with the size of the left atrium of the heart.11,17,18 This angle may be widened due to heart disease (enlarged left atrium, cardiomegaly and pericardial effusion) and mediastinal abnormalities (subcarinal masses),1,3 while it may be reduced after pulmonary lobectomy and lobar collapse.1 The tracheal angle was also correlated with the size of the left atrium of the heart.11,17,18
As the carina located approximately at the level of T5 and T6 vertebrae during expiration in inspiration, because of this proximity to the spine increases the bifurcation angle. This data can be useful for calculating dead space. Another clinical importance would be regarding posture in patients with suppurative pulmonary disorders.9
The literary context points to the importance of the contribution of anatomical knowledge of the tracheobronchial tree for the early diagnosis and treatment of respiratory diseases and malformations, including fetal tracheobronchial surgery in the womb. Thus, a good knowledge of the biometric parameters of the fetal airway is necessary to determine the criteria for adaptation to the fetal and neonatal tracheobronchial anatomy,14 with consequent improvement in the management of the procedure.
The present study aimed to establish, in human fetuses, in situ, through morphometry, the dimensions and quantifications of the tracheal angles, relating them with gestational age and gender.
An observational and cross-sectional analytical study was conducted at the Anatomy and Forensic Investigation Research Center of the Tiradentes University. The fetuses belong to the Anatomy Laboratory of Tiradentes University and were obtained in accordance with the Brazilian Law No. 8501 of 1992, which deals with the use of unclaimed corpses for studies and research. For research, the fetuses were selected taking into account the gestational age - established from the rump length measured with tape and later comparison with fetal growth table.5 Thus, 41 fetuses of both sexes, aged between 20 and 38 weeks, were selected, classified and maintained in 10% formaldehyde solution. These fetuses selected according to gestational age were divided into four groups for the study (Table 1).
|
Groups by gestational age |
Number of fetuses |
Division by |
|
|
I.20–24 weeks |
8 |
5 |
3 |
|
II.25–28 weeks |
19 |
8 |
11 |
|
III.29–32 weeks |
8 |
4 |
4 |
|
IV.33–38 weeks |
6 |
2 |
4 |
|
Total of fetuses (20–38 weeks) |
41 |
19 |
22 |
Table 1 Groups of fetuses according to gestational age
F, female; M, male
The anterior region of the neck and thorax of each fetus was opened through a midline incision from the upper edge of the thyroid cartilage to the level of the middle third of the thorax. In this way, they were exposed and dissected to the naked eye: the trachea, its bifurcation and the right and left main bronchi. The tracheobronchial angles (TBA), right (TBAr) and left (TBAl) were evaluated in situ, through the acquisition of images, from which, using the Angulus software, digital analyzes were performed (Figure 2). Measurement values in degrees and corresponded to TBArTBAl formed by passing two axes: one vertical, the point of bifurcation of the trachea and the other by the lower edge of the corresponding main bronchus. The TBA was also calculated, which corresponds to the intersection between the lower edges of the main bronchi. The values found were tabulated and analyzed using the following statistical tests: Mann-Whitney, Kruskal-Wallis, ANOVA, and Spearman correlation. All variables were compared with gestational age in order to verify if there is an increase in angles according to age, as well as their correlation with gender. A significance level of p<0.05 was also considered for the statistical tests used.
With a 0.01mm precision caliper, the following tracheal measurements were also performed: total length (TL); proximal transverse external diameter (PTED); and distal transverse external diameter (DTED) (Figure 3). The number of cartilage rings was also counted (NCR). Except NCR, different researchers measured all other variables twice -, and the average of the two measures were considered for statistical analysis. All values found were analyzed and correlated with tracheal angles.
In Table 2 are shown are shown by gender, in degrees, the mean and standard deviation of TBA, and TBAr, TBAl. In this table, we can still see that, regardless of the age group of the fetuses, there was no statistically significant difference between the mean TBA, TBAr and TBAl. Despite not having been significant statistics among the media, it can be seen that all averages TBAr were slightly lower than the TBAl.
|
Gender |
|||||||
|
Total |
Female |
Male |
|||||
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
p-value |
|
|
TBA |
63.50º |
19.10 |
61.29º |
14.98 |
65.24º |
21.97 |
0,895 |
|
TBAr |
26.42º |
10.24 |
23.75º |
5.94 |
28.50º |
12.37 |
0,431 |
|
TBAl |
37.09º |
10.64 |
37.54º |
10.28 |
36.73º |
11.13 |
0,618 |
Table 2 Correlation of fetal tracheobronchial angles by gender
SD,standard deviation; Mann-Whitney test; TBA, tracheobronchial angle; TBAr, tracheobronchial right; TBAl, tracheobronchial left
Table 3 shows the findings regarding the values of means and standard deviation of tracheobronchial angles in relation to gestational age. One can observe a statistically significant difference between the means of the ATB. This may indicate that this measure was influenced by gestational age. Meanwhile, no statistically significant differences were found between TBAr and TBAl.
|
20 to 24 weeks |
25 to 28 weeks |
29 to 32 weeks |
33 to 38 weeks |
||
|
Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
p-value |
|
|
TBA |
51.59º (11,9) |
65.84º (15,76) |
76.68º (26,66) |
54.45º (14.35) |
0,032 |
|
TBAr |
20.76º (5,11) |
26.36º (7,59) |
34.81º (16,23) |
22.95º (6.94) |
0,103 |
|
TBAl |
30.83º (8,62) |
39.48º (10,42) |
41.86º (10,87) |
31.5º (9.3) |
0,111 |
Table 3 Correlation between fetal tracheobronchial angles by gestational age
SD, standard deviation; Kruskal-Wallis test; TBA, tracheobronchial angle; TBAr, tracheobronchial right; TBAl, tracheobronchial left
Table 3 also shows that there was an increase in mean angle values according to gestational age. This increase occurred between 20 and 32, which was subsequently followed by a decrease in the mean value of angles in gestational age fetuses between 33 and 38 weeks. However, there was statistical significance for ATB. Regardless of age, the TBAr was always less than the TBAl.
ANOVA was used to compare angles and the relationship between gestational age and fetal sex. Table 4 shows the difference of averages were not statistically significant, although it was close to p<0.05 when correlated with gestational age.
|
Gestational Age |
Gender |
Gestational Age * Gender |
|
|
F (p-value) |
F (p-value) |
F (p-value) |
|
|
TBA |
2,493 (0,077) |
0,018 (0,893) |
0,824 (0,490) |
|
TBAr |
2,311 (0,094) |
0,829 (0,369) |
0,812 (0,496) |
|
TBAl |
2,268 (0,099) |
0,358 (0,554) |
0,656 (0,585) |
Table 4 Correlation between fetal tracheobronchial angles by age and gender
F, statistics F; ANOVA; TBA, tracheobronchial angle
The NCR ranged from nine to 22, with a mean of 16 and standard deviation of cartilage 2.26. When comparing this variable with the TBA, by age and sex, it seems that there is no significant differences (p>0.05), as shown in Table 5. Although not significant, it is noteworthy that the coefficient of determination (R²) was greater than 0.9, indicating a strong correlation between ATB - by gestational age and gender - and the number of cartilages. It is possible that this has occurred due to the small sample size, which may have been a limiting factor in this study. Considering that R² as a measure that described the cartilage count, by sex and gestational age, it could be explained from this correlation that the higher the number of cartilages, the higher the TBA.
|
Total tracheobronchial angle |
|||||
|
Source |
Sum of Squares |
DF |
Medium Square |
F |
p-value |
|
Cartilage Count |
299,847 |
1 |
299,847 |
0,909 |
0,348 |
|
Gestational Age |
1,928,666 |
3 |
642,889 |
1,949 |
0,143 |
|
Gender |
7,692 |
1 |
7,692 |
0,023 |
0,880 |
|
Gestational Age * Gender |
701,259 |
3 |
233,753 |
0,709 |
0,554 |
|
Error |
9,895,852 |
30 |
329,862 |
||
|
Total |
174819,930 |
39 |
|||
Table 5 Correlation between number of tracheal cartilage and total tracheobronchial angle
R²=0.943 (Adjusted R²=0.926). DF, degrees of freedom; F, statistics
Figure 4 shows Spearman's correlation between ATB, ATBd, and ATBe with TL, DTED, and PTED. The data are showing that there was no correlation between the value of tracheal angles and their linear variables. As the Spearman coefficient (P), approaching 0 was a random or no apparent relationship between morphometric data and tracheal angles. No significant correlation (p>0.05).
This study was an analysis and interpretation, as a whole, the values of three tracheal angles (TBA, and TBAl TBAR) in 41 fetuses aged 20 to 38 weeks. The sample in this study was greater than that found in other studies with human fetuses.8,11,12 Moreover, as the normality assumption is not guaranteed, non-parametric tests were used not to increase the rate of false significant results.
Similar to other studies,8,11,12 there was no lack of interrater variability for all numerical data were controlled by a single investigator. On the other hand, a software was used to measure the three angles tracheobronchial and held a semi-automatic evaluation of these angles through digital images.
Findings of the present study were similar, in discrepancies, described as to the values of the tracheal bifurcation angle regarding gender11,12,19 and age.4,11,20 The ATB ranged from 30.8º to 128.6º, with an average of 63.5º. While the TBAR ranged from 11.7º to 67.2º, with an average of 26.4º and the TBAl from 17.7º to 61.4º, and an average of 37.1º. n findings reported by Daroszewski11 ATB varied 36,2º to 96,6º, mean ±73,1º 12,7º. The TBAR ranged from 11.4º to 41.8º, with an average of 26.9º±7.0º and the TBAl ranged from 24.8º to 64.8º with an average of 46.2º±8.0°.
The results obtained in this study are inconsistent with those described by Karabulut et al.18 and Murray et al.21 On the other hand, they agree with those reported by other authors,4,8,11,13,15,20–23 who also found no significant differences between the tracheobronchial angles to the gender. Angles larger tracheobronchial found in women13,18 would possibly due to obesity.18
Similar to what has been described by many authors, the findings in this study show that, regardless of gender and age, the right bronchial angle was smaller than the left.8,11,12,19,20,24
It was also observed an increase in angles between 20 and 32 weeks and, on the contrary, a decrease between 33 and 38 weeks. The averages for total TBA were statistically significant, suggesting that the measurement may be influenced by gestational age. The increase of such angles according to the age of the fetuses is in agreement with the results obtained by some authors,8 but not among others.11,18 The literature does not seem to have registered, until now, the decrease of tracheobronchial angles, as found in the present study. However, narrowing of tracheobronchial angles has been recorded in the pediatric population as age increases.13
As we age, the tracheobronchial angle narrows and approaches the midline, making the right bronchus almost a continuation of the trachea. In older children and adults, this occurrence causes a predilection for foreign body aspiration directly into the right main bronchus.13 This corroborates the present study, where it was found that the average of TBAr was lower than those of TBAl. Some authors25,26 emphasized the greater airflow through the right lung to the left carina position. Others have shown that the distance from the carina to the column was inversely correlate the tracheobronchial angles.18 In this study, chest shape was not correlated with tracheal angles. However, some studies reported that a weak inverse correlation occur.11,12,18 However, it was found that tracheobronchial angles - regardless of age and gender - did not correlate with the number of cartilages, nor with the proximal TL, PTED, and DTED. No correlation seems to be described in the literature.
Since intubation, bronchoscopic intervention or the provision of medical equipment for airway management in children are performed differently compared to adults, the knowledge of the tracheobronchial anatomy becomes important for physicians in their thoracic approaches.13 Thus, the present findings may represent an important knowledge to endotracheal intubation, procedures in the tracheobronchial tree, pulmonary physiology studies and anthropometry pure.8,13
The main limitation of this study may have been the small number of investigated tracheas with relatively narrow gestational ages.
None.
We declare no conflict/competing of interests.
None.

©2020 Almeida, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.