MOJ
eISSN: 2471-139X


Review Article Volume 10 Issue 1
Department of Anatomy, Faculty of Basic Medical Science, Bingham University, Nigeria
Correspondence: Elijah S, Department of Anatomy, Faculty of Basic Medical Science, Bingham University, Karu, Nigeria
Received: June 07, 2023 | Published: November 17, 2023
Citation: Elijah S, Abraham A, Tabitha A. Ameliorative effects of Buchholzia coriacea seed on aluminium chloride induced cytotoxicity in the liver of adult male wistar rats. MOJ Anat Physiol. 2023;10(1):42-46. DOI: 10.15406/mojap.2023.10.00337
Buchholzia coriacea seeds has been reported to have various medicinal properties. This study evaluates the effect of the aqueous extract of Buchholzia coriacea seed (wonderful kola), on Aluminium chloride induced liver toxicity in adult male Wistar rats. 24 rats weighing between 90g-200g were divided into five groups. Group A- (the control group) received water and animal chow. Group B received 200mg/kg of AlCl3 only, Group C received 200mg/kg body weight of aqueous extract Buchholzia coriacea only. Group D received 50mg/kg body weight body weight (Low Dose) of aqueous extract Buchholzia coriacea and (200mg/kg) AlCl3. Group E received 250mg/kg body weight (High Dose) of aqueous extract of Buchholzia coriacea and (200mg/kg) AlCl3. The animals were sacrificed, the liver was excised and processed for routine paraffin sections at 5micromes thick. Sections were stained with Haematoxylin and Eosin to demonstrate histoarchitecture. Biochemical analysis was also carried out to determine the effects on liver antioxidant enzymes. Histological assessment showed narrowed central vein, degeneration of hepatocytes and distortion of sinusoids in the AlCl3 alone treated groups. The histoarchitecture of the liver in the other groups showed improvement especially in Group E (High Dose of B. coriacea) and Group C (B. coriacea extract only). The biochemical analysis on the other hand showed significantly reduced activities in GSH, SOD, CAT and significantly increased level of MDA in the Alcl3 only group. In the other groups, MDA levels reduced, GSH, SOD and CAT also increased enzymatic activities following the administration of B. coriacea. The study concludes that Buchholzia coriacea seed extract plays a role in protecting liver from Alcl3 toxicity.
Keywords: aluminium chloride, cytotoxicity, liver, Coriacea seed, male wistar rats
Aluminium is considered one of the ubiquitous metals on earth, ranked as the third most abundant after oxygen and silicon. The environmental level of Aluminium has increased tremendously by Crisponi1 et al., thanks to intensive growth in Aluminium industries Brough and Jouhara.2 Aluminum, present in many manufactured food and medicines has the potential to be toxic to human and animals. It is also added to drinking water in the course of purification, thus increasing human exposure to this toxic metal.3 Aluminum accumulates mainly in bone, liver, kidney, testis, and brain resulting to biological dysfunction.4 It enters the body through respiratory and gastrointestinal tracts and accumulates in different tissues including the liver causing hepatoxicity.5 Buchholzia coriacea Eng. (Capparaceae), also known as musk tree, named after R.W. Buchholz,6 has edible seeds with a characteristic sharp and pungent smell with a hot spicy taste.7The seed crude powder and its solvent extracts were reported to have radical scavenging and protective potentials against experimentally-induced hepato-renal injury and have high amounts of flavonoid which is said to help avoid widespread diseases such as cancer, inflammation, atherosclerosis, ischemic injury and neurodegenerative diseases by contributing along with antioxidants, vitamins and enzyme to the total antioxidant defense system in the human body.7 Phytochemical constituents of Buchholzia coriacea also include alkaloids, anthraquinones, cardiac glucosides, saponins and tanins.8,9 B.Coriacea Seed is a medicinal seed reputed for several biological activities like having anthelmintic,10 antimicrobial ,11 hypoglycemic,7 anti-inflammatory12 and cognitive enhancing effects.8 The free radical scavenging activities of B. Coriacea Seed have also been reported by various authors.13,14 Hence, this study evaluates its effect on aluminum chloride induced toxicity on the liver of Wistar rats.
Plant preparation and extraction
The seeds were grated into small pieces, dried and pulverized into coarse powder. Extraction was done via cold maceration. One kilogram of the plant material was extracted in distilled water for 72 hours with intermittent shaking at 2h interval. The extract was then filtered using Whatman No. 1 filter paper. The filtrate was concentrated using rotary evaporator (Rota vapor R 210, Büchi, Switzerland) at 40°C and then lyophilized using a freeze dryer. The yield was determined and the extract stored in a refrigerator at 4°C in sample containers prior to use.
Procurement and breeding of animals
Twenty-five presumably healthy adult male wistar rats weighing between 90g-200g were used for this study. These animals were procured from Nigerian Institute of Veterinary Research (NIVR) located in Vom, Jos, Plateau State. Upon arrival, the animals were acclimatized for 14 days. They were housed in well ventilated cages at room temperature in hygienic condition under natural light and dark schedule in the Animal House of the Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, Bingham University, Karu, Nasarawa state, Nigeria. These animals were maintained on a regular common rat feed (Vital feed growers mash). Food and water were given spontaneously.
Chemical and reagents- Analytical Pure crystalline Aluminium chloride Anhydrous was obtained from xilong chemical, China with CAS number (6153-566) 126.07 .
Ethical clearance/approval- Ethical approval for the study was sort for and gotten from the ethical clearance committee of Bingham University with approval number BHU/ETC/APP/034.
Experimental protocol- The experiment was carried out within the period of 28 days. Animals were divided into five groups with five (5) rats one cage. The aqueous extract of Buchholzia coriacea (50mg/kg and 250mg/kg b. wt) and Aluminum chloride (200mg/kg b.wt) were dissolved in distilled water. Proper concentrations were administered orally with the use of metal oropharyngeal cannula for twenty-eight (28) days with respect to their body weight.
Group A: Animals served as control and were given rat feed and water only for 30days
Group B: Animals received 200mg/kg b. wt of wonderful kola only.
Group C: Animals received 200mg/kg b. wt of Aluminum Chloride only.
Group D: Animals received stock solution (Low dose 50mg/kg b.wt) of wonderful kola and 200mg/kg b.wt of Aluminum Chloride.
Group E: Animals received stock solution (high dose 250mg/kg b. wt) of wonderful kola and 200mg/kg b. wt of Aluminum Chloride. All extracts were water soluble hence were aqueous extracts.
Animal sacrifice and liver tissue collection - The animals were sacrificed by sedating them with chloroform and the abdominal region was dissected to expose the liver, which was excised. The liver specimen was washed in normal saline and fixed in a specimen bottle. The liver tissue for histological analyses was fixed by using 10% formalin as the fixative in the specimen bottle. The liver tissue for biochemical assay was fixed by using potassium Chloride as fixative.
Histological analysis and photomicrography
Pieces of the liver were fixed and embed in 10% formalin, Sections of 5 μm thickness were obtained using a rotary microtome before staining using haematoxylin and eosin. Histological slides were viewed using a digital microscope with objective lens x 400. Slides micrograph were viewed and photomicrographically taken at objective lens x 40.
Biochemical analysis- Tissue samples from liver were taken for determination of values of liver antioxidant; malondialdehyde (MDA), reduced glutathione (GSH) levels, and the activities of Superoxide dismutase (SOD) and catalase (CAT).
Estimation of MDA: This method depends on the formation of MDA as an end product of lipid peroxidation which reacts with thiobarbituric acid producing thiobarbituric acid reactive substance (TBARS), a pink chromogen, which can be measured spectrophotometrically at 532 nm, an MDA standard was used to construct a standard curve against which readings of the samples were plotted.
Estimation of GSH: The method is based on the reduction of 5,5 dithiobis (2-nitrobenzoic acid) (DTNB) with reduced glutathione (GSH) to produce a yellow compound. The reduced chromogen is directly proportional to GSH concentration and its absorbance can be measured at 405 nm by using a commercial kit.
Determination of tissues CAT- It is assayed by the method of Sinha which based on formation of chromic acetate from dichromate and glacial acetic acid in presence of hydrogen peroxide, chromic acetate that was produced and measures colorimetrically at 570 nm, one enzyme unit was defined as the amount of enzyme which catalyzed the oxidation of 1 μmole H2O2 per minute under assay conditions.
Estimation of SOD activity-The activity of hepatic SOD was assayed using a commercially available SOD Activity Assay Kit (Biovision, K335-100), according to the manufacture’s instruction. Statistical analyses were performed with 2-way ANOVA using GraphPad Prism 8. Differences were considered to be of statistical significance at an error probability of P<0.05.
Figures 1–9.
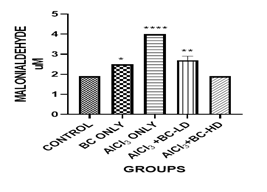
Figure 1 Graph show the values for the Glutathione after 4 weeks of treatment with Aluminium Chloride and B. coriacea seeds (wonderful kola).
There was statistically significant difference between the groups P < 0.05.
KEY: BC, Buchholzia coriacea; AlCl3, Aluminium Chloride; BC-LD, Buchholzia coriacea – low dose; BC-HD, Buchholzia coriacea – high dose
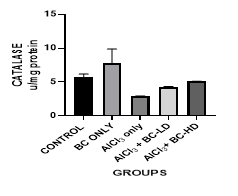
Figure 2 Graph show the values for the liver catalase enzymes after 4 weeks of treatment with Aluminium Chloride and B. coriacea seeds (wonderful kola). There was a no statistically significant difference between the groups P < 0.05.
KEY: BC, Buchholzia coriacea; AlCl3, aluminium chloride; BC-LD, Buchholzia Coriacea – low dose; BC-HD, Buchholzia coriacea – high dose
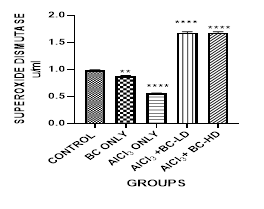
Figure 3 Graph show the values for the liver superoxide dismutase enzymes after 4 weeks of treatment with Aluminium Chloride and B. coriacea seeds (wonderful kola). There was statistically significant difference between the groups P < 0.05.
KEY: BC, Buchholzia coriacea; AlCl3, aluminium chloride; BC-LD, Buchholzia coriacea – low dose; BC-HD, Buchholzia coriacea – high dose
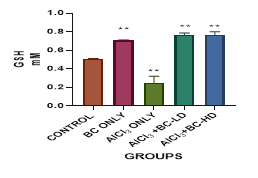
Figure 4 Graph show the values for the Glutathione after 4 weeks of treatment with Aluminium Chloride and B. coriacea seeds (wonderful kola). There was statistically significant difference between the groups P < 0.05.
KEY: BC, Buchholzia coriacea; AlCl3 , aluminium chloride; BC-LD, Buchholzia coriacea – low dose; BC-HD, Buchholzia coriacea – high dose
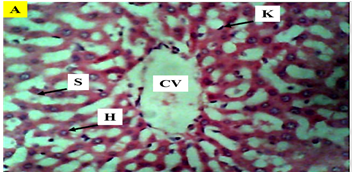
Figure 5 Photomicrograph (×400) of hematoxylin and eosin stained liver section of Control group A, showing hepatocytes and nucleus within normal cellular architecture.
KEY: H, hepatocytes; CV, central vein; K, kupffer Cells; radially arranged; S, sinusoids
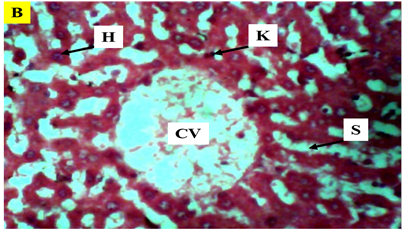
Figure 6 Photomicrograph (×400) of hematoxylin and eosin stained liver section of Group B treated with BC only) reveal normal histological appearance.
KEY: H, hepatocytes; CV, central vein; K, kupffer Cells; radially arranged; S, sinusoids
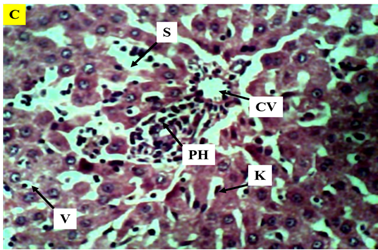
Figure 7 Photomicrograph (×400) of hematoxylin and eosin stained liver section of Group C treated with 200mg/kg AlCl3 only, reveal narrowing of central vein, pyknotic hepatic nuclei, vacuolation and sinusoidal ballooning.
KEY: H, pyknotic hepatocytes; narrow CV, central vein; K, kupffer Cells; enlarged radially arranged S, sinusoids; V, vacuolation
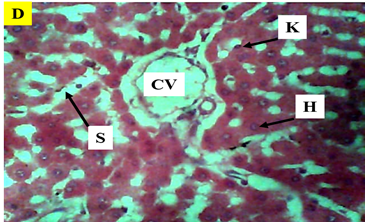
Figure 8 Photomicrograph (×400) of hematoxylin and eosin stained liver section of Group D treated with 200mg/kg AlCl3 and 50mg/kg Buchholzia Coriacea seeds reveal that co-administration of aqueous extract of Buchholzia Coriacea seeds had some modulatory effect on the Aluminium chloride induced alterations.
KEY: H, hepatocytes, CV, central vein, K, kupffer Cells, radially arranged S, sinusoids
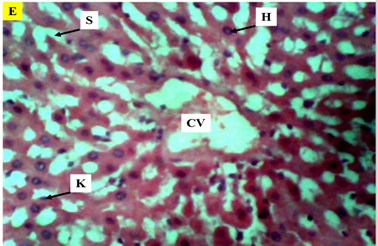
Figure 9 Photomicrograph (×400) of hematoxylin and eosin stained liver section of Group E treated with 200mg/kg AlCl3 and 250mg/kg Buchholzia coriacea seeds showing the protective effect of Buchholzia coriacea seeds on AlCl3 induced hepatic degeneration in rats but with congested central vein.
KEY: H, hepatocytes; CV, central vein; K, kupffer Cells; radially arranged S, sinusoids
The average mean value for Malondialdehyde (MDA) in the Aluminium Chloride group is higher and statistically significant when compared with the other groups. The level of GSH was lowest in the Aluminium Chloride-alone -treated group. It was statistically significant when compared with control group and other groups. The administration of the B. coriacea seeds significantly increased the level of GSH. The level of Superoxide Dismutase (SOD) was also significantly decreased in the Aluminium Chloride only treated group. The administration of B. coriacea seeds increased the levels of SOD in all treated groups. The mean values for Catalase (CAT) in all the groups are not statistically significant except for the B. coriacea seeds, and Aluminium Chloride (AlCl3), only groups. Representative images (×400) of hematoxylin and eosin stained formalin-fixed paraffin-embedded liver section, showing the protective effect of Buchholzia coriacea seeds on AlCl3 induced hepatic degeneration in rats.
The seed extract of B. coriacea is reported to be hepatoprotective and to also possess antioxidant properties.13 Studies have also shown that the hepatotoxic effect of aluminium chloride is mediated by the generation of free radicals via oxidative stress,15 Hence, the present study investigated the protective effects of B. coriacea against Aluminium Chloride induced toxicity in the liver of adult male wistar rats via biochemical analysis of antioxidant enzymes and histological analysis of the liver.
Oxidative stress is the disturbance that exists between the balance in free radical generation and antioxidative enzymes and molecuoles, in favor of radical production.16 Lipid peroxidation is a known hallmark of oxidative stress. The free radical mediates a chain reaction which results in oxidative deterioration of polyunsaturated lipids with malondialdehyde (MDA) as one its major toxic by -products. Accordingly, malondialdehyde is used as a biomarker of oxidative stress induced lipid peroxidation. The level of lipid peroxidation increased by the aluminum chloride administration clearly shows an imbalance between pro-oxidant and antioxidant system, which automatically induces oxidative stress. The increase in MDA in the liver, as recorded in the present study, could be due to the resultant increase in the production of free radicals like hydrogen peroxide and hydroxyl radicals in the liver of aluminum chloride treated rats. The value for MDA in the ALCL3 group is higher and statistically significant than the other groups. The increased lipid peroxidation as a result of AlCl3 administration is in line with the observation of other authors.13,15 The aqueous seed extract of Buchholzia coriacea may have attenuated the AlCl3 induced increase in liver homogenate concentrations of MDA due to its antioxidant potential.14,17
In this present study elevated levels of malondialdehyde (MDA) but reduced levels of glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) in the liver samples of aluminum chloride (AlCl3) -treated rats was recorded. The significant decrease recorded in the activities of liver antioxidant protein and enzymes (GSH, SOD and CAT) reflect the failure of antioxidant defense system to overcome the influx of ROS precipitated by AlCl3 administration. These observations are in accordance with Kumar, et al.,18 who observed significant drop-in activities of SOD, GSH and CAT after AlCl3 treatment. The glutathione peroxidase system comprises of several components, one of which is GSH. GSH, is a very essential component of the oxidative system. It serves as a cofactor for glutathione transferase, which is known to help in the removal of certain drugs, chemicals and other reactive molecules, from cells by Ebrahim and Kambiz. Moreso, GSH can interact directly with certain ROS, i.e., hydroxyl radicals to detoxify them, as well as performing other critical activities in the cell. Hence, GSH may probably be the most important antioxidant molecule present in cells. In our study GSH was lowest in the ALCL3 treated group, but it increased significantly in the groups involving B. coriacea seeds (wonderful kola) administration. Aluminum might also affect GSH synthesis by decreasing glutathione-synthase activity, which would result in reduced GSH levels.5 A study carried out by Ore et al19 showed significant amelioration of liver function markers and levels of pro-inflammatory proteins by Hydroethanolic extract of defatted Buchholzia coriacea seeds (HEBCS). Histopathological studies showed a reduction in inflammatory cells and improvement in liver structure in animals treated with HEBCS. Hydroethanolic extract of defatted Buccholzia coriacea seeds (HEBCS) also protected against High Fat Diet-induced inflammation, oxidative stress and hepatocellular damage.20
The enzymatic antioxidant defense system, which includes SODs and CATs, can decompose superoxide and hydrogen peroxide in the cells and are the main defense against oxidative injuries. SOD catalyzes the rapid removal of superoxide radicals, generating H2O2. Therefore, SOD works in collaboration with H2O2 removing enzymes.21 The level of Superoxide dismutase (SOD) also decreased significantly in the ALCL3 only treated groups, and like GSH the administration of B. coriacea increased the levels of SOD. CAT is present in the peroxisomes of nearly all aerobic cells and functions to protect the cell from the toxic effects of hydrogen peroxide through catalyzing its decomposition into molecular oxygen and water without the production of free radicals Al-Hashem.4 In this study the values for catalase (CAT) in all the groups are not statistically significant except for the Wonderful Kola and Aluminium Chloride, only groups. Also, treatment with the extract was able to ameliorate altered liver function.
Parameters of malaria parasite: infected mice
Also, treatment with the extract was able to ameliorate altered liver function
Parameters of malaria parasite: infected mice treatment with the extract was able to ameliorate altered liver function
Parameters of malaria parasite: infected mice.
The photomicrographs obtained from the present research shows the cytoarchitecture of the liver in different groups as displayed by Haematoxylin and Eosin stains. The histological results of H and E stain showed the cytoarchitecture of the normal liver indicative of the control Group A, showing the sinusoidal spaces with normal hepatic plates with well distributed Kupffer cells in between the plate. The group that received Aluminium chloride showed narrow central vein, degeneration of hepatocytes and distortion of sinusoids. This is in line with previous studies Co-administration of aluminium chloride with Buchholzia coriacea seeds mitigated the deleterious effects of aluminium chloride in groups D and E. 22,23 This is in agreement with the study by 24 where the histological assessment showed that B. coriacea mitigated neurodegenerative changes, this suggests the hepato-protective efficacy of Buchholzia coriacea seeds. The results from this study demonstrated that treatment with B. coriacea only protects the liver. The protective effect of B. coriacea could be attributed to its antioxidant activity. B. coriacea contains phytochemicals such as flavonoids, Saponins, alkaloids etc.8,9 Flavonoids are shown to have antioxidative activity, scavenging capacity, and hepatoprotective ability. This is in agreement to research conducted by Enechi et al.,9 on the ameliorative effects of flavonoid-rich extract of B.coriacea seeds on modifications in hematological and biochemical status induced by malaria in mice.25–28
Limitation of the study
This study did not assess lipid profile and liver function test.
The present study has provided supportive evidence that the oral administration of Aluminium chloride in male rats at a dose of 200 mg/kg body weight daily for a period of 30 days induces hepatic dysfunction as evidenced in significant alterations in some biochemical and histological parameters. The use of aqueous extract of Buchholzia coriacea seeds in combination with Aluminium chloride was observed to attenuate some of the harmful effects of this element. Therefore, supplementation with Buchholzia coriacea seeds may prove useful as protective therapy against the hepatotoxic effects of Aluminium chloride.
None.
There is no conflicts of interest.

©2023 Elijah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.