MOJ
eISSN: 2471-139X


Research Article Volume 7 Issue 5
1Department of Medical Biology, Cihan University, Iraq
2College of Dentistry, Babylon University, Iraq
3Department of General Biology, Cihan University, Iraq
Correspondence: Homady MH, College of Science, Department of Medical Biology, Cihan University, Erbil, Kurdistan region, Iraq, Tel +9647517118678
Received: October 18, 2020 | Published: October 27, 2020
Citation: Homady MH, Alquraishi LO, Hussein AM, et al. Affinity of liver cells to Sudanophilia and PAS in castrated treated mice. MOJ Anat Physiol. 2020;7(5):155-160. DOI: 10.15406/mojap.2020.07.00307
The present study was conducted on seventy five healthy males of Swiss albino mice, which were divided into 5 groups (N=15 mice per each group). The first group were intact (control); the second one were castrated; the third group were castrated treated with 50µg/kg testosterone; the forth one were castrated treated with 10µl/g. grape juice, and the fifth one were intact treated with 3mg/kg F. hormonis.
The frozen sections ofmice liver tissue from both castrated and intact treated subjects with 3mg/kg Ferula hormonis showed a strong affinity to sudanophilia (+++) as compared with the intact (control) group. Whereas frozen sections from castrated subjects treated with 50µg/kg of testosterone or with10µl/g of grape juice showed a moderate affinity to sudanophilia (++) as compared with the control. The histological sections of liver tissue from castrated group or intact treated with 3mk/kg F. hormonis revealed a decrease in glycogen contents as compared with the control group. On the other hand, sections from castrated subjects treated with 50µg/kg of testosterone or with 10µl/g of grape juice appeared normal appearance to intact (control) subjects.
Keywords: sudanophilia, liver, lipids, glycogen, ferula hormonis
The mammalian liver is the largest internal digestive organ, morphologically and functionally is a complex digestive gland, which is indispensable in many essential physiologic processes and vulnerable to be impaired by a wide variety of factors, such as toxins, microorganisms, metabolic products, circulatory materials and metabolism of carbohydrates, lipids and proteins.1,2 It is made up of the largely predominant parenchymal cells (hepatocytes), and also non-parenchymal cells, including Ito (perisinusoidal cells), Kupffer cells and sinusoidal endothelial cells.3,4
Surgical castration is a bilateral (excision of both testes) removal of testis, resulted in depletion of androgen.5,6 Farm animals have been castrated to eliminate breeding and reduce aggressive behavior.7,8 Hypogonadism after castration caused abdominal obesity, impaired fasting glucose, excess accumulation of liver triglyceride, and thigh muscle loss. Castration also induced increase of visceral fat mass and decreased thigh muscle mass in mice.9 Castration is a way of studying the consequences of extreme testosterone deficiency in animal models.10 Testosterone suppresses ER stress, inhibits the formation of macrovesicular lipid droplets, promotes lipid export, and ameliorates steatohepatitis induced by castration.11
The primary and most well-known androgen is testosterone which is the product of dihydrotesterone (DHT). Serum testosterone levels decline gradually and progressively with aging in men. Low levels of testosterone are associated with metabolic disorders, including obesity, dyslipidemia, hypertension, and insulin resistance.12–15 The degree of insulin resistance correlates negatively with serum testosterone16,17 and low serum testosterone levels have a higher risk of developing hepatic steatosis.18
The anti-fertility activities of medicinal plants have been extensively investigated in mice and rats.19 Several species of these plants were reported to have estrogenic activity while few of them show significant contraceptive properties.20 Phytoesterogenic are novel esteron found in a variety of plants which may be ingested directly as constituent tissues from animals that have digested plant phytoestrogen, have noxious effect leading to infertility in domestic animals as well as disturbance of normal gestation processes. There are a number of phytoestrogen plants likely to be consumed by human such as Ferula hormonis, the toxic effect of Ferula in cattle is associated with hemorrhage disease probably due to ferulenol, fepernin, 4-hydroxylated, and prenylated coumarins.20 Some medicinal plants may exert promising pharmacological properties and improve the effectiveness of conventional medications as complementary agents.21
Vitisvinifera (Grape) are one of the most consumed fruits globally. It possesses a wide range of pharmacological activities due to its rich polyphenol ingredients most of which have been demonstrated to have therapeutic or health promoting properties,22–24 among them, flavonoids are the most abundant and widely studied, recent studies have shown that the beneficial health effects promoted by consumption of grape and grape products are attributed to the unique mix of polyphenolic compounds. As the largest group of grape polyphenols, flavonoids are the main candidates considered to have biological properties, including but not limited to antioxidant, anti-inflammatory, anti-cancer antimicrobial, antiviral, cardio protective, neuroprotective and hepatoprotective.25–27 Therefore the present study is aimed to study the affinity of hepatocytes to sudanophilia and to determine the quantity of glycogen contents in hepatocytes cells in different surgical and physiological conditions such as:-castration, castration treated with testosterone, or grape juice as well asin intact animal treated with F. hormonis.
Swiss albino male mice weighting between (14-17) g., and aged (3weeks) were used in the present study, the mice were obtained from the Animal House, Faculty of Science/ University of Kufa. Animals were kept in ventilated cages, with a temperature of (25±2ºC) at 12:12h light, dark cycle was used balanced, rodent food pellet and water were provided ad libitum.28 All experimental protocols using live animals were first reviewed, approved and accepted according to guidelines for the care and use of laboratory animals in biomedical research.29,30
A total number of 75 Swiss albino mice were used in the present study. Animals were divided into 5 groups (N=15), and the treatment was started at the age of 21 days for 6 weeks as:
Group I: Intact male mice received tap-water as control.
Group II: Castrated male mice received tap-water as (positive group).
Group III: Castrated male mice treated daily with 50µg/kg testosterone.
Group V: Castrated male mice treated daily with 10µl/g. grape juice,
Group IV: intact male mice treated with 3mg/kg F. hormonis.
The surgical castration method was done according to31
Testosterone dose Selection: A previous study documented that 50µg/kg/day of the testosterone solution was not toxic,31 for the present protocol, 50µg/Kg/day was used in the present study. This dose is given daily as intramuscularly on consecutive thigh muscle for six weeks.
Plant material extract
Black grape (Vitisvinifera) obtained from local market (Baghdad, Iraq) 100g of grape was blundered by using a commercial blender without separating the seeds, and then it was filtered to remove the residue. The resulting extract (10mls) was stored in the refrigerator at 4ºC., and used after one hour. The extract was prepared according to.32 A previous study documented that 10µl/g/day of grape juice extract was effective dose.33–35 For this protocol, we used in the present study 10µl/g/day and was given daily as orally administered for six weeks.
Ferula hormonis
Roots were obtained from a local market in (Amman, Jordan), the extract material was prepared according to the procedure described by32 In the course of the experiments (3g) of the residue was dissolved in 100mls of distilled water and 3mg/kg were used.
Animals were sacrificed at the end of the experiments, with using ketamine and xylazine as anesthetic drugs to anesthetize the mice.The preparation procedures for frozen sections and Periodic Acid Schiff (PAS) have been described by.32 The frozen sections were stained by using Sudan black B.
Digital analysis
Digital analysis was performed using image J software. Digital of lipid droplets in frozen section stained with Sudan black B.36,37 Results were expressed as the mean ± standard deviation of the mean (S.D). Data for multiple variable comparisons were analyzed by one-way analysis of variance (ANOVA). For the comparison of significance between groups, Duncan's test was used as a post hoc test according to the statistical package program (SPSS version 16.0) the p values P<0.05 was considered as significant for all statistical analysis in this study.
The present results of Table 1 show the scoring number of Sudanophilia in different experimental groups. Figure 1 representing frozen sections from intact (control) male mice stained with Sudan Black B showing a weak affinity to Sudanophilia (+).Whereas sections from both castrated and intact subjects treated with 3mg/kg F. hormonis (Figure 2 & 3) showed a strong affinity to Sudanophilia (+++) as compared with control group. On the other hand frozen sections from castrated subjects treated with 50µg/kg testosterone or castrated treated with 10µl/g grape juice (Figure 4&5) showed a moderate affinity to Sudanophilia (++) as compared with control.
|
A (percentage of positive cells) |
B (intensity of staining) |
score (multiplication of A and B) |
Power |
|
0 = no positive cells |
0 = no color reaction |
0-1 = negative |
0 poor |
|
1 = <10of positive |
1 = mild reaction |
2-3 = mild |
+ weak |
|
2 = 10-20 positive cells |
2 = moderate reaction |
4-8 = moderate |
++moderate |
|
3 = 21-50 positive cells |
3 = intense reaction |
9-12 = strongly positive |
+++ strong |
|
4=>50 |
Final IRS score (A × B): 0-12 |
Table 1 Showing the scoring number of Sudanophilia in different experimental groups of male mice
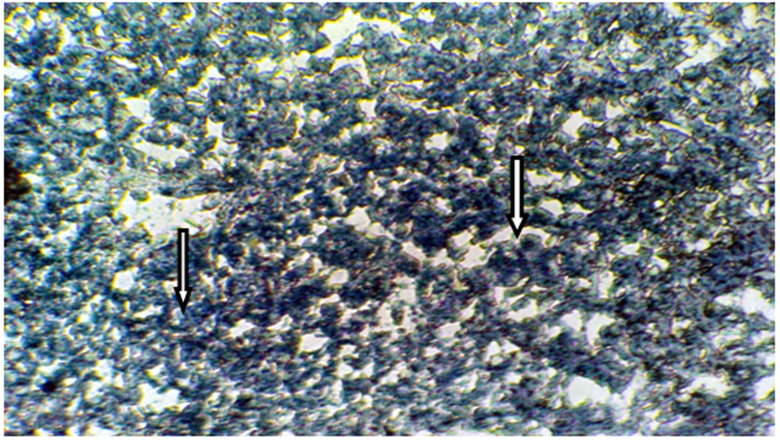
Figure 1 Frozen section of liver tissue from intact male mice stained with Sudan Black B showing a weak affinity to Sudanophilia (+), arrow indicated lipid droplets (X400).
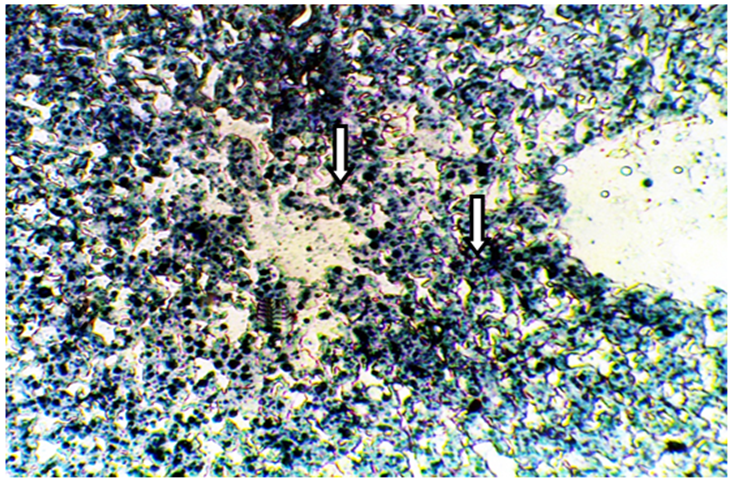
Figure 2 Frozen section of liver tissue from castrated male mice showing a strong affinity to Sudanophila (+++), arrow indicated lipid droplets (X400).
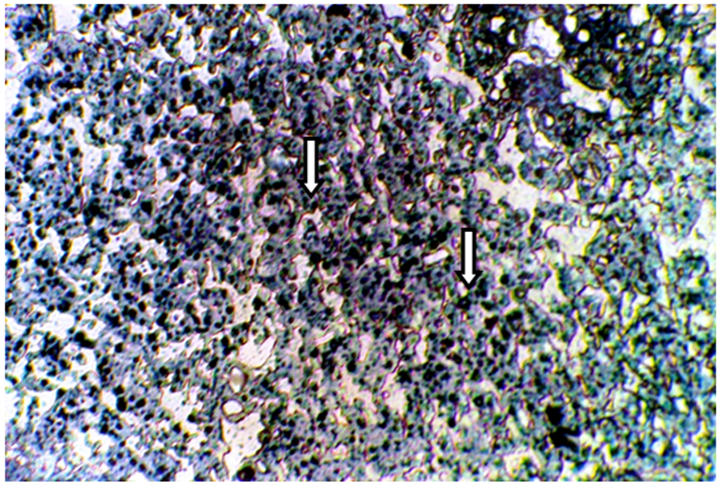
Figure 3 Frozen section of liver tissue from intact mice treated with 3mg/kg F. hormones stained with Sudan Black B showing a strong affinity to Sudanophilia (+++), arrow indicated lipid droplets (400X).
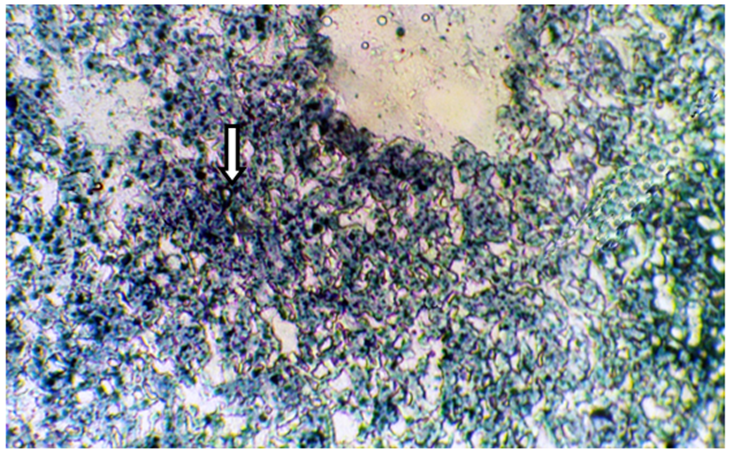
Figure 4 Frozen section of liver tissue from castrated subject treated with 50µg/kg testosterone showing a moderate affinity to Sudanophilia (++), arrow indicated lipid droplets (X400).
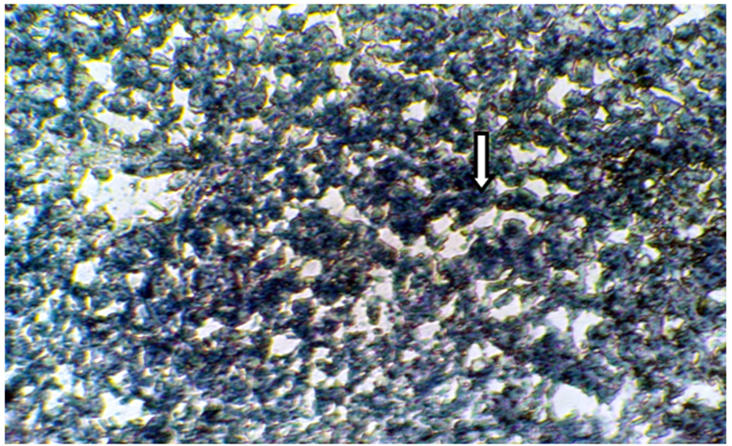
Figure 5 Frozen section of liver tissue from castrated mice treated with10 µl/g grape juice showing a moderate affinity to Sudanophilia (++), arrow indicated lipid droplets (X400).
Liver glycogen detection: By using Periodic Acid Schiff (PAS) stain the histological sections of liver tissue from intact male mice (Figure 6) showed a normal glycogen contents. However, the histological sections of liver tissue from castrated (Figure 7) and intact group treated with 3mg/kg of F. hormonis (Figure 8) appeared a decrease in the amount of glycogen contents as compared with the control subject. Whereas the histological sections from castrated group treated with 50µg/kg testosterone (Figure 9) as well as, castrated group treated with 10µl/g grape juice (Figure 10) showed return the glycogen contents to the normal state as compared with the control group.
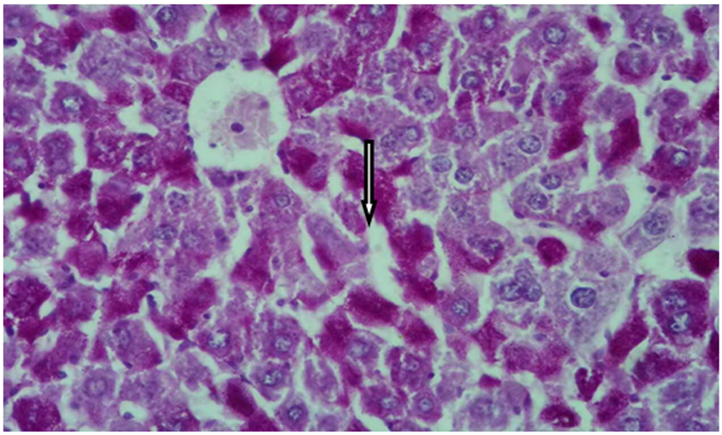
Figure 6 Cross histological section of liver tissue from intact male mice Stained with PAS showing normal glycogen contents, arrow indicated glycogen contents (X400).

Figure 7 Cross histological section of liver tissue from castrated group stained with PAS showing a decrease in glycogen contents (X400).
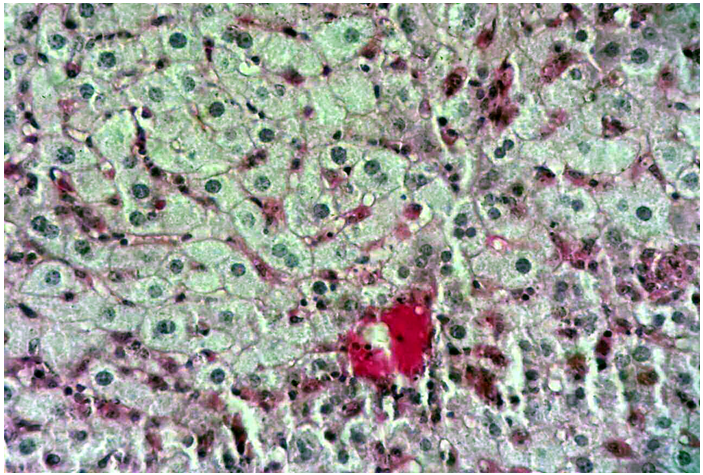
Figure 8 Cross histological section of liver tissue from intact group treated with 3 mg/kg F. hormones stained with PAS showing a decrease in glycogen contents (X400).
The present findings of liver frozen sections from castrated group showed a strong intensity (+++) to Sudanophilia. These results can be attributed to the accumulation of intrahepatic lipids, which were mainly triglycerides. Frozen section of liver tissue from castrated group treated with testosterone showed a moderate intensity (++) to Sudanophilia , these results may be due to the ameliorate role of testosterone in increasing number of Sudaniphilia through decreasing B-oxidation, free radicals and hepatic lipogenesis. It has been stated that low doses of testosterone in impairment functions, lead to accumulation of lipids with large number of Sudanophilia insides the hepatocytes.38 the present findings are in agreement with the findings of39–41 who concluded that liver cells increased their affinity to Sudanophilia after treatment with testosterone.Also these findings are in concomitant with the findings of42 who stated thatthere was a significant increase in testosterone level in castrated subject treated with testosterone that resulted in an increase of Sudanophilia of liver cells.
The results of liver tissue from castrated group treated with grape juice showed a moderate intensity (++) to Sudanophilia. These results may be due to the protective role of grape juice by increasing number of Sudanophilia through reducing the free radicals, B-oxidation that lead to increase the Sudanophilia in hepatocytes and return them to normal function and structure.
On the other hand frozen sections of liver tissue from intact group treated with F. hormonis showed a strong intensity (+++) to Sudaophilia. These findings can be attributed to many factors: it might be due to anti androgenic activity of F. hormonis that inhibit the testosterone level in the tissues which lead to increased adipose lipolysis and hepatic lipogenesis. However, F. hormonis may have direct action on the gluconeogenesis or disruption of normal process within either the hypothalamic pituitary axis or gonads.43 Reported that subjects treated with F. hormonis extract resulted in a testicular tuberculosis and suppression of spermatogenesis. Studies by44,45 are also in agreement with these findings who showed that intragastric administration of F. hormonis resulted in several pathological effect such as: colon adenocarcinoma, decrease of blood elements, and reduction in body weight, decline in social aggression, abnormalities of sperm and many other marked physiological and biochemical disruption.
According to the present findings of glycogen contents from castrated group showed decreased amount of glycogen contents. These results are in agreement with the findings of46 who stated that, there is a relationship between low level of testosterone and insulin sensitivity, increased hyperglycemia lead to decrease in glycogen contents in skeletal muscle and liver.On the other hand insulin resistance was confirmed by the decreased expression of key regulator genes of glucose up- take and utilization in muscle of androgen deficient in castrated mice as compared to the control groups. The present findings are in agreement with,47 who concluded that, testosterone deficiency caused adiposity, insulin resistant, B-pancreatic cells failure, increased lipolysis and hepatic lipogenesis with decreased glycogen contents.
The histological sections from castrated group treated with testosterone revealed a normal intensity to glycogen contents. This result may be due to those metabolic effects of testosterone and regulating FAS level in subcutaneous tissue also decreased adipose lipolysis which considered the main source in hepatic steatosis.48,49 reported that testosterone replacement in castrated animals maintain the glycogen contents within normal value. These findings are in agreement with others,47,50 who stated that testosterone replacement in castrated rate restore the castration effects and glucose homeostasis.
The findings of liver sections from castrated group treated with grape juice also showed a normal glycogen contents these results may be due to the protection effects of grape juice against oxidative stress that was caused to increase glutathione and catalase in the liver tissues and/or grape juice may has an antioxidant and free radical scavenging activities, therefore protecting against oxidative stress and replenishing the reduced glutathione contents. This administration of grape juice ameliorated and enhanced the antioxidant defense against castration induced oxidative stress. These results are in agreements with the findings of,51,52 concludedthat the potential beneficial role of grape juice in preventing oxidative stress-mediated damage and strengthening antioxidant defense mechanisms, with an increase in the antioxidant status of animals.
Histological sections of liver tissue from intact group treated with F. hormonis showed a decrease in glycogen contents. These results may be due to the anti-androgenic effects of F. hormonis and its role in impairment the glycogen contents through conversion all the glucose to lipids by impairment in glycogenesis which cause to decrease the glycogen contents. To our knowledge there was no research work has been conducted to evaluate the effect of F. hormonis on theliver tissue and these findings may be due to that cholestasis or biliary obstructive in bile duct might play some pathogenic role indecreasing glycogen contents. The present results are in agreement with,43 reported that the intragastric application of F. hormonis extract caused inhibition of normal growth in testes and other male sexual organs. As well as, this treatment caused cystic swelling and tuberculosis in the testes.
From the achieved results of this study, we can conclude the following: Both castration and F. hormonis have reversible effects on liver cells. Through increasing their affinity to Sudanophilia as well as decreasing their glycogen contents. On the other hand testosterone and/or grape juice have dramatic effects for restoring liver cells to normal structure in castrated subjects.
None.
The authors declare there are no conflicts of interest.
None.

©2020 Homady, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.