MOJ
eISSN: 2471-139X


Research Article Volume 4 Issue 4
1Faculty of Medical Sciences, National University of Cuyo, Argentina
2Nuclear Medicine School, Argentina
3Faculty of Medical Sciences, National University of Cuyo-CONICET, Argentina
Correspondence: Fernando D Saravi, Instituto de Fisiologia, Facultad de Ciencias Medicas, Universidad Nacional de Cuyo, Avenida del Libertador 50, Mendoza, MZ 5500, Argentina, Tel 542614135006, Fax 542614614000
Received: July 27, 2017 | Published: November 3, 2017
Citation: Saraví FD, Ruiz MIG, Carra GE, et al. Additive effect of chronic hypoxia and aldosterone on electrogenic sodium transport in rat rectal epithelium. MOJ Anat Physiol. 2017;4(4):331–335. DOI: 10.15406/mojap.2017.04.00142
Background: Both chronic hypoxia and mineralocorticoids independently stimulate electrogenic sodium absorption in rat distal colon. It is hitherto unknown whether there is any interaction between these two stimuli. The aim of this work was to assess the interaction of these stimuli on rat rectal epithelium in vitro.
Methods: Isolated rectal mucosa samples were obtained from adult rats submitted to hypobaric hypoxia for 10 days or breathing at normal pressure. Tissues were mounted in Ussing chambers which allow simultaneous measurement of short-circuit current (ISC) and oxygen consumption rate (QO2). Both variables were measured at baseline, after 8 h-incubation with aldosterone (10 nM) and after addition of the epithelial sodium channel, amiloride. Statistical analysis was performed with Student’s t tests and linear regression.
Results: At baseline, samples from hypoxic rats had higher ISC (p=0.0002) but its QO2 did not differ from the control group (p=0.289). Incubation with aldosterone caused a progressive increase of ISC in both groups, but the difference in ISC between them was maintained throughout the incubation period. At the end of the incubation period, both groups showed significant increases in ISC and QO2. Amiloride reduced ISC and QO2 to the same extent in both groups. QO2 and ISC were linearly related to each other both at the baseline and at the end of the incubation period.
Conclusion: While the rectal epithelium of chronically hypoxic rats had a higher ISC, its response of incubation with aldosterone at nanomolar concentration was preserved and followed the same time course than that of the rectal epithelium of non-hypoxic rats. Chronic hypoxia and aldosterone showed an additivity of effects in the rectal epithelium. The increase in ISC and QO2 caused by hypoxia and aldosterone was abolished by amiloride, suggesting an ENaC-dependent response to both stimuli.
Keywords: aldosterone, amiloride, chronic hypoxia, oxygen consumption, rectal epithelium, sodium channel, ussing chamber
ENaC, epithelial sodium channel; ISC, short-circuit current; QO2, oxygen consumption rate
A remarkable functional feature of the intestinal epithelium is its normally low oxygen pressure, a condition that has been dubbed “physiological hypoxia”.1 The colonic epithelium reabsorbs about 90% of the water contained in the chime flowing through the ileocecal valve and modifies its electrolyte composition, with net sodium and chloride absorption and net potassium and bicarbonate excretion.2 Fecal dehydration is closely linked to sodium and chloride absorption, because the latter provides the driving force for the former.2,3
Although sodium and chloride absorption occurs throughout the colon, the precise mechanisms are different along the various colonic segments.3 There are also differences between species. For example, in the rabbit and human distal colon, sodium absorption is an electrogenic process, dependent on apical epithelial sodium channels (ENaC) which are sensitive to low concentrations of amiloride.4‒6 However, in the rat distal colon, sodium absorption is normally insensitive to amiloride, occurring as an electroneutral process dependent on coupled apical Na+/H+ and Cl-/HCO3- exchangers.7‒9 Nevertheless, ENaCs are expressed at the apical membrane of the rat distal colon epithelium.10,11
The colonic epithelium is, like other sodium transporting epithelia, a classical target for aldosterone and related mineralocorticoid hormones.12 In the rat distal colon, mineralocorticoids not only increase net sodium absorption but also cause a switch in its underlying mechanism, from electroneutral to electrogenic, mediated by functional ENaCs. This change may be brought about by increased endogenous secretion of aldosterone in response to a low-sodium diet,13 by administration of natural or synthetic mineralocorticoids,14 and also by incubation of the epithelium with physiological concentrations of aldosterone in vitro.15 This aldosterone-induced electrogenic sodium transport is associated with increased epithelial oxygen consumption, and both effects are blocked by amiloride.16
On the other hand, chronic hypobaric hypoxia also induces electrogenic sodium transport in the rat distal colon,17 although it does not increase QO2.18 It is highly unlikely that this response is mediated by mineralocorticoids, since chronic hypoxia actually inhibits aldosterone synthesis19 and dramatically lowers plasma aldosterone levels.a href="#ref18">18,20
Since both aldosterone and chronic hypoxia induce electrogenic sodium transport, the present work aimed at determining whether the acute response to aldosterone of the late distal colon epithelium is preserved, blunted or increased in chronically hypoxic rats.
Animals
The experimental protocol of this study was designed according to the National Institutes of Health guidelines for animal research. It was reviewed and approved by the Committee for Animal Care and Biosafety of our Medical School.
Adult Wistar-Hokkaido male rats weighing 250 to 300g were used in all the experiments. Rats were brought from the Medical School animal facility and housed in the same room at 24ºC with a 12/12h light/dark cycle. Control rats were kept at ambient pressure (about 92kPa, or 0.91atm, in Mendoza), while rats submitted to chronic hypoxia were placed two at a time, in wire mesh-bottom cages, 8 hours per day during 10days in a high flow 150-L hypobaric chamber with an inner pressure of 51kPa (about 0.5atm), roughly equivalent to an altitude of 7000m above sea level. The chamber was slowly re-pressurized and opened each day for cleaning and feeding purposes.
Animals were fed on a standard pelletized diet for rodents with a sodium content of 5g/kg (0.22mmol/g) and a caloric content of 3.5kCal/g (Cargill Co., Buenos Aires, Argentina). Previous work has shown that ad libitum food consumption in chronically hypoxic rats is about 75% of the food consumption of control rats, which in turn is approximately 80g/day per kg of body weight.16 To avoid possible confounding differences caused by lower electrolyte intake3 or lower intake of nutrients that may affect colonic ion transport, like protein21 or fatty acids,22 rats of both groups were given 60g/day per kg of body weight. Free access to distilled water was allowed to both groups.
Assessment of systemic response to hypoxia
Trunk blood was withdrawn during the surgical procedure. An aliquot of blood was anticoagulated with calcium heparine (Calciparine, Sanofi-Aventis, Buenos Aires, Argentina). An aliquot of this blood was employed for determination of hematocrit according to a standard microtechnique. The rest of the heparin-treated blood was centrifuged at 1000rpm for 30minutes to obtain a plasma sample. Sodium concentration was measured in plasma with a model 84-11 Orion Ross sodium electrode connected to a model 720 A meter (Orion Research, Inc., Beverly, Massachusetts, USA). The remaining blood was allowed to clot and its serum was frozen for later determination of aldosterone concentration with a coated-tube radioimmunoassay (Diagnostic Products Corporation, Los Angeles, California, USA).
Gas, solution, and drugs
For all experiments, we used a mixture of 95 % O2 and 5 % CO2 , with its composition analyzed and certified by the provider (Air Liquide, Inc., Buenos Aires, Argentina). The Ringer solution had the following composition: 132.8mM Na; 4.5mM K; 1.25mM Ca; 1.0mM Mg; 114mM Cl; 24mM HCO3; 0.8mM HPO4; 0.2mM H2 PO4; 10mM D-glucose; 0.5 mM b-hydroxybutirate; 2.5mM glutamine and 10mM D-mannose.16 The pH of the solution was 7.40 when gassed to saturation. Aldosterone and amiloride were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Gentamicin was obtained from Schering-Plough (Buenos Aires, Argentina). It was added to the Ringer solution at a final concentration of 91mg/mL to prevent bacterial over growth. Aldosterone was dissolved in absolute ethanol and amiloride in dimethyl sulfoxide to yield final concentrations in the chamber of 10nM and 0.1mM, respectively. At the added volumes, neither absolute ethanol (10mL) nor dimethyl sulfoxide (25mL) had any measurable effect on short-circuit current (ISC) or QO2.
Surgery, dissection, and mounting
All experiments were routinely started between 7 AM and 8 AM. Under ether anesthesia (10%), animals were killed by opening the thorax. The abdomen and the pelvic bones were cut open and the whole colon, from the cecum to the anus, was removed and rinsed free of contents. Segments for study were obtained from the pelvic portion of the colon, beyond a lymph node located at the pelvic brim, also called “late distal colon”16 or “rectal colon”.23,24 The micro dissection technique has been described before.17 The segment was dissected to obtain an isolated mucosa preparation, which was cut open and gently stretched. Its adherent mucus layer was carefully removed with a cotton tip soaked in Ringer solution; then the sample was mounted as a flat sheet in a modified Ussing chamber.
Using chamber
The modified Ussing chamber used in the experiments reported here has been previously described.25 The chamber is hermetic and has an opening of 1cm2. Each hemichamber has a bubble trap which allows the injection of drugs and a port for inserting a polarimetric oxygen probe (CellOx 325) connected to a WTW Oxi 340 oxygen meter (WTW GmbH, Weilheim in Oberbayern, Germany). The probes permitted continuous measurement of oxygen concentration and temperature in both hemichambers. A small polytetrafluoroethylene-coated magnetic bar was placed at the bottom of each hemichamber for continuous mixing of its contents by a magnetic stirrer (HI 300N, Hannah Instruments, Woonsocket, Rhode Island, USA). During the experiments, the chamber temperature was kept at 37.0ºC by an inbuilt water jacket.
Electrical measurements
Transepithelial potential difference was recorded with calomel electrodes connected to each hemichamber through 3% agar-in-Ringer bridges. An amplifier with correction for bridge asymmetry and solution resistivity allowed passing current through Ag/AgCl2 electrodes. The experiments were performed clamping the potential difference at 0mV, with continuous monitoring of current in a digital display and recording in a paper chart recorder under the short-circuit condition, except for brief periodical releases to measure open circuit potential difference.
Determination of oxygen consumption rate
Oxygen meters were calibrated according to the user’s manual, and their slopes were checked before each experiment. Taking into account chamber volume and oxygen solubility in Ringer at 37ºC, oxygen consumption rate (QO2) was calculated from the rate of change in oxygen concentration in each hemi chamber, taking into account chamber volume and oxygen solubility at 37ºC. In order to check the air-tightness of the chamber, blank runs were performed placing a polyethylene membrane instead of the biological sample. The spontaneous rate of decrease of the oxygen concentration was always less than 5% of that observed during measurements of baseline epithelial QO2.
Experimental procedures
The chamber was filled with Ringer and gassed with 95% O2 and 5% CO2 to saturation and afterwards closed. A 30min period was allowed for equilibration, after which baseline QO2 was measured for 30min. This was followed by addition of aldosterone to both hemichambers. To avoid too large decreases in oxygen concentration at the end of the experiment the chamber was opened twice during the aldosterone incubation period, gassed with humidified 95% O2 -5% CO2 and closed again. Preliminary experiments showed that this procedure per se had no effect on epithelial oxygen consumption when the oxygen concentration in the chamber was above 5ppm before gassing. After 7h, with the chamber closed, QO2 was measured by a second 30min period. Then amiloride was added to the mucosal hemichamber and 20min later a third 30min QO2 measurement was performed.
Statistical methods
Statistical analysis was performed with Prism for Windows, version 5.04 (GraphPad Software, Inc., San Diego, California, USA). A one-way analysis of variance (ANOVA) for repeated measures was used for comparing the effect of treatments (aldosterone and amiloride) within each group. Comparisons between both groups were done by a two-sided Student’s t test for unpaired samples with Welch’s correction. D’Agostino and Pearson omnibus normality test was performed to check for significant deviations from a Gaussian distribution. Linear regression analysis, with a check for significant deviation from linearity, was used for assessment of the relationship between short-circuit current and oxygen consumption in each group and for comparing the slopes and elevation of the regression lines of each group. Results are expressed as mean±SEM unless stated otherwise. Differences were deemed statistically significant at P<0.05.
Systemic adaptation to hypoxia
The body weight of control rats (n=12) was initially 275.3±5.0g. It was 273.6±4.2g after 10days of restricted intake (p=0.567). The initial body weight of rats submitted to hypoxia (n=12) was 278.6±4.4g. It decreased to 261.5±3.8g after 10days of exposure (p <0.0001). Initially, there was no significant difference between groups (p=0.619), but hypoxic rats reached a slightly lower body weight than control rats after 10days (-12.1±5.7g; p=0.045). The weight loss of hypoxic rats was mostly accounted for a reduced food intake during the first three days of exposure to low ambient oxygen pressure. At the time of surgery, the hematocrit of control rats was 42.7±0.4%, while that of hypoxic rats was 61.7±0.5% (p<0.0001). Plasma sodium concentration was 142.1±0.4 mmol/L in control rats and 141.2±0.8mmol/L in hypoxic rats (p=0.325). Serum aldosterone concentration was 1.41±0.21nmol/L in the control group and 0.45±0.12nmol/L in the hypoxic group (p=0.0007).
Short-circuit current and oxygen consumption rate
At baseline, before incubating the samples with aldosterone, the ISC, of epithelia was higher in hypoxic rats than in controls (respectively, 4.12±0.75mmol.h-1.cm-2 vs 2.95±0.38 mmol. h-1.cm-2; p=0.0002), while no difference was found in QO2 (respectively, 2.85±0.32 mmol. h-1.cm-2 vs 2.73±0.21 mmol.h-1.cm-2; p=0.289). A plot of QO2 vs ISC shows a significant linear correlation for both groups. The regression coefficient r was 0.814 for the control group (p=0.0013) and 0.939 for the hypoxic group (p<0.0001). The slope was non-significantly higher in the hypoxic group than in the control group: 2.215±0.225 vs 1.454±0.328 (p=0.0889). However, the elevation of regression line of the hypoxic group was significantly higher (p<0.0001) (Figure 1).
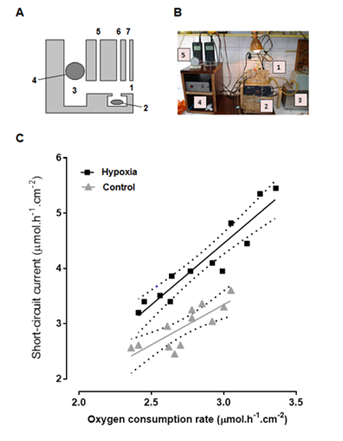
Figure 1 A, diagrammatic lateral view of the Ussing chamber employed for measuring oxygen consumption rate (1, window; 2, magnetic stirrer; 3, main reservoir; 4, oxygen probe; 5, current inlet; 6, bubble trap, and 7, inlet for Agar bridges). B, experimental set up (1, Ussing chamber; 2, magnetic stirrer apparatus; 3, thermostatic bath; 4, amplifier, and 5, oxygen meters). C, Linear regression of oxygen consumption rate vs short circuit current at baseline; r was 0.814 for the control group (p =0.0013) and 0.939 for the hypoxic group (p < 0.0001). There was no significant difference between slopes (p = 0.0889). The elevation of regression line of the hypoxic group was significantly higher (p <0.0001).
The time course of ISC with aldosterone incubation in both groups is shown in Figure 2. Short-circuit current was higher in epithelia from hypoxic rats than in those from control rats at all times, except after addition of amiloride, but the time course of the changes in ISC was similar for both groups. The results of QO2 measurements and the corresponding values of ISC during the same periods are shown in Table 1.
Baseline |
Aldosterone |
Amiloride |
|
Short-Circuit Current (mmol.h-1.cm-2) |
|||
Control (n = 12) |
2.95 ± 0.11 |
7.85 ± 0.12§ |
0.24 ± 0.06§ |
Chronic hypoxia (n = 12) |
4.12 ± 0.21* |
8.90 ± 0.30#§ |
0.40 ± 0.12§ |
Oxygen Consumption Rate (mmol.h-1.cm-2) |
|||
Control (n = 12) |
2.71 ± 0.07 |
3.51 ± 0.08§ |
2.32 ± 0.06§ |
Chronic hypoxia (n = 12) |
2.85 ± 0.08 |
3.79 ± 0.09¶ § |
2.25 ± 0.09§ |
Table 1 Effects of hypoxia, aldosterone and amiloride on short-circuit current and oxygen consumption of isolated rat rectal mucosa
*:p = 0.0002 compared with control; §: p > 0.0001 compared with baseline; #: p = 0.0056 compared with control; ¶: p = 0.0298 compared with control
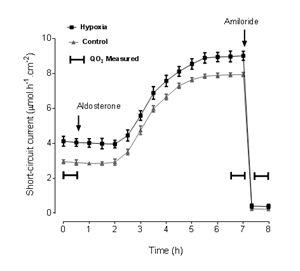
Figure 2 Time course of short-circuit current upon incubation with aldosterone and the effect of amiloride. The periods of oxygen consumption measurement are indicated.
At the second QO2 measurement, after aldosterone incubation and just before adding amiloride, QO2 and ISC were significantly higher than at baseline in both groups (p<0.0001). Additionally, both QO2 (p=0.0298) and ISC (p=0.0056) were significantly higher in epithelia from the hypoxic group. These differences were abolished after addition of amiloride.
Relationship between QO2 and short-circuit current
The relationship between the decrease in QO2 and the decrease in ISC at the end of the aldosterone incubation period and after amiloride addition is shown in Figure 3. In both cases, there was a significant linear relationship with r=0.927 for the control group and r=0.896 for the hypoxic group. The slopes of the regression lines were, respectively, 2.205±0.281 and 1.798±0.282; p=0.322). In this case, no difference was noticed in the elevation of the regression lines (p=0.362).
As previously reported, distal colon epithelium obtained from rats chronically submitted to hypoxia had a higher ISC than controls,17 but baseline QO2 was not significantly different between both groups.19 Instead, at baseline, epithelial samples from hypoxic rats had a higher ISC for each value of QO2.19 This increase in ISC without a proportional increase in QO2 may represent an adaptive response to decreased oxygen availability, allowing normal sodium absorption even with low aldosterone serum levels.
Since a tight coupling between electrogenic transport and QO2 has been found in this epithelium.18,19,25 and the ISC of the distal colon epithelium from chronically hypoxic rats remains as sensitive to acute hypoxia as that from controls,17 a partial switch to anaerobic metabolism seems an unlikely explanation for the higher ISC in hypoxic rats. Hence, the larger ISC in epithelia from chronically hypoxic animals might be explained either by the use of a larger fraction of totals QO2 for electrogenic transport, or by a better coupling between aerobic metabolism and ionic transport. Obviously, these two hypotheses do not exclude each other. However, the larger decrease of QO2 with electrogenic transport blockade in hypoxic rats supports the first hypothesis, namely that a larger fraction of total QO2 is employed for electrogenic transport during chronic hypoxia.
While rectal epithelia from rats fed on a high sodium diet do not show an amiloride-sensitive ISC, rectal epithelia of rats fed on a low sodium diet, or even a normal sodium diet, do show a significant amiloride-sensitive ISC.19 In the present study, both groups were fed on a normal sodium diet, and their response to incubation with aldosterone was qualitatively similar and had the same time course. Furthermore, at the end of the incubation period, amiloride reduced ISC and QO2 to the same extent in both groups. Before incubation with aldosterone, the regression line of QO2 vs ISC had a significantly higher elevation in samples from hypoxic rats, indicating a higher ISC for each value of QO2 for tissues from chronically hypoxic rats. However, at the end of the period of incubation with aldosterone, the elevation of the regression lines of both groups did not differ between them. This may be due, at least in part, to the fact that in the latter case changes in ISC and QO2 were plotted, instead of absolute values. It is also possible that the large effect of aldosterone incubation overshadows the smaller effect of chronic hypoxia.
The physiological implications of the present study are threefold. First, it indicates that chronic hypoxia enhances sodium absorption in the rat rectal epithelium by a mechanism which may partially compensate for the hypoxic decrease in aldosterone secretion. Second, it shows the rectal epithelium form chronically hypoxic rats retain the ability to respond to exogenously administered aldosterone. Third, the additivity of chronic hypoxia and aldosterone effects suggests that the increase in electrogenic sodium transport caused by both stimuli follows different signaling paths.
The main limitation of the present study is that the responses to chronic hypoxia and aldosterone have been assessed only at the tissue level. Further studies at the single cell level, like for example electrophysiological patch-clamp recording, and genomic and proteomic analysis of chronically hypoxic cells, will be needed for a complete characterization of the response and a deeper understanding of the underlying pathways and mechanisms.
The rectal epithelium of chronically hypoxic rats has a higher rate of electrogenic transport with no change in oxygen consumption at baseline. However, its response of incubation with aldosterone at nanomolar concentration is preserved and follows the same time course than that of the rectal epithelium of non-hypoxic rats. The effect of aldosterone was larger than the effect of chronic hypoxia, but both treatments showed additivity of their effects in the rectal epithelium. The increase in ISC and QO2 caused by hypoxia and aldosterone was abolished by amiloride, suggesting that both treatments share a common ENaC-dependent response.
The authors gratefully thank Ms. Liliana M Cincunegui for excellent technical support. The participation of María Isabel González Ruiz in this study was made possible by a research scholarship of the Institute of Basic Sciences, National University of Cuyo. The work was supported by grant 06/J487 from the Secretariat for Science, Technology and Postgraduate Studies of the National University of Cuyo.
The authors declare that they have no competing interests.

©2017 Saraví, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.