MOJ
eISSN: 2471-139X


Delivery of blood to bodily tissues and organ is the responsibility of the arterial side of the circulatory system. The inner most component of the vascular wall that is in contact with circulated blood is a layer of cells known as the endothelium. Normally, endothelial cells interact with blood in a fashion that influences the smooth muscles of the vascular wall and prevents adhesion of platelets and other circulating cells. Such interactions involve production and release of substances that maintain vascular diameter or dilate it. Healthy endothelium ensures proper patency of vessels and, consequently, proper tissue perfusion. The purpose of this review is to briefly explain the role of healthy endothelium, causes of endothelial dysfunction, assessment of endothelial dysfunction, and the consequences of such dysfunction in relation to cardiovascular disease. This review also explores aspects of maintaining a healthy endothelium and remedies for the reversal of its dysfunction.
Keywords: atherosclerosis, cholesterol, coronary disease, endothelium, heart failure, hypercholesterolemia, hypertension
EDRF, endothelium-derived relaxing factor; NO, nitric oxide; EDCF, endothelium-derived contracting factor; EH, essential hypertension; HOPE, heart outcome prevention evaluation
Cardiovascular disease remains to be the main cause of death in developed and developing countries. Although the cause of cardiovascular disease remains unknown, it is now clear that an impairment of tissue perfusion represents the primary problem. Three main factors contribute to the impairment of tissue perfusion, which are: enhanced vasoconstrictor responses, increased interaction of circulating blood cells and structural changes of the arterial intima. Anatomically, the endothelium is the inner most layer of the vascular wall that is in contact with circulating blood and is adjacent to the smooth muscles of the wall. The influence of the endothelium on vascular smooth muscle and other circulatory functions was not known until nearly three decades ago. The role that the endothelium plays in changing vascular tone and in cardiovascular disease became recognized in 1980.1 Currently, it is realized that healthy endothelium is important in maintaining normal blood flow and in many blood and cardiovascular events. Risk factors such as smoking, hyperlipidemia, diabetes mellitus and hypertension contribute to endothelial dysfunction. This review involves various aspects of the endothelium in relation to cardiovascular disease.
Physiology of the endothelium
Normal endothelium releases a substance in response to neurotransmitters, hormones, substances derived from platelets and the coagulation system that causes relaxation of the vascular smooth muscle. Also, shear forces that are generated by circulating blood induce vasodilation as an adaptive response to exercise. Such was characterized as being endothelium-dependent and the released substance became known as the endothelium-derived relaxing factor (EDRF). Later on, this EDRF was identified as nitric oxide (NO), a free radical that has a half-life of a small number of seconds. It was found that oxidation of the guanidine-nitrogen terminal of L-arginine produces NO.2 The enzyme system that is involved in synthesizing NO was found in endothelial cells, vascular smooth muscle cells, nerve cells as well as in circulating platelets and macrophages.3 An increase in cyclic 3’, 5’-guanosine monophosphate (cGMP) in vascular smooth muscle, via the soluble enzyme guanylyl cyclase, results because of NO-induced vascular relaxations.4 Nitric oxide can also increase cGMP in platelets, via the action of guanylyl cyclase, which in turn reduces adhesion and aggregation. Endothelial cells also release prostacyclin, which increases cyclic 3’, 5’-adenosine monophosphate (cAMP) in smooth muscles and platelets. Prostacyclin and NO act synergistically to inhibit platelet aggregation – thus, suggesting that both are required for maximal inhibition of platelet aggregation. As endothelial cells can cause vasodilation, they are also involved in vasoconstriction. The endothelium produces a number of substances known as endothelium-derived contracting factors (EDCF), which include: endothelin-1 (ET-1), thrombaxane A2, prostaglandin H2, and angiotensin 2. It was shown that ET-1 causes vasodilation at lower concentrations – however marked and sustained contractions were attained at higher concentrations.5 Endothelium-derived vasoconstrictors can be produced through the cyclooxygenase pathway. Substances such as arachidonic acid, acetylcholine, histamine and serotonin can evoke endothelium-dependent contractions that are mediated by thrombaxane A2 or prostaglandin H2. Also, the endothelium was shown to play an important role in regulating the activity of the rennin-angiotensin system. The vascular relaxing and constricting factors released by the endothelium are shown in Table 1. A simple diagram showing the balance between vaso-dilating and vaso-constricting factors in normally-functioning endothelium is presented in Figure 1.
S. No |
Relaxing Factors |
Contracting Factors |
1 |
Endothelium derived nitric oxide |
Cyclooxygenase dependent endothelium derived constricting factors |
2 |
Prostacyclin (PGI2) |
Endothelia 1 |
3 |
Endothelium-derived hyperpolarizing factor (EDHF) |
Thrombaxane A2 |
4 |
Atrial natriuretic peptide |
Prostaglandin H2 |
5 |
Adrenomedulin |
Angiotensin 2 |
Table 1 Relaxing and constricting factors released by endothelium
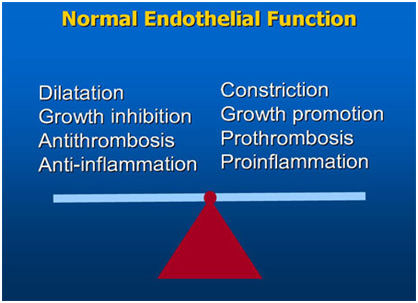
Figure 1 The balance between vaso-dilating and vaso-constricting factors ought to be maintained for normal endothelial function.
Endothelial dysfunction
An alteration in the balance between the contrasting endothelium-derived contracting and relaxing factors results is endothelial dysfunction. Such imbalance constitutes factors that can be considered as risks for cardiovascular disease. Endothelial dysfunction and atherosclerosis are common in epicardial coronary arteries and large arteries such as the aorta and iliac artery. Rupture of atherosclerotic plaque and endothelial cell denudation (damage) are the most common causes of acute coronary syndrome. Also, such morphological changes in the endothelium are most likely related to functional alterations and intimal layer thickening.
Endothelial dysfunction in hypertension
Hypertension is associated with functional and morphological alterations of the endothelium.6 In hypertensive blood vessels, endothelial cells have an increased volume and bulge into the lumen. The sub-intimal space exhibits structural changes with increased fibrin and cell deposition. And the interaction of endothelium with platelets and monocytes is increased in hypertensive subjects compared with normotensives. Studies have shown that patients with essential hypertension (EH) have impaired response to acetylcholine, an endothelium-dependent vasodilator7,8 and a normal response to sodium nitroprusside, an endothelium-independent vasodilator. These results indicated that patients with EH have a specific deficit in the endothelium-derived NO system and this defect could partly be responsible in determining both the increased vascular resistance and the impaired response to endothelium-dependent agents in these patients. It has been shown that blunted endothelium-dependent vasodilator responses in essential hypertensive subjects is mainly due to a reduced NO activity9 and not because of decreased availability of L-arginine,10 or because impaired responsiveness of the vascular smooth muscle to nitro-vasodilators.7,8 Impaired endothelial function was found in normotensives subjects with a family history of hypertension11 as well as in the off spring’s of EH patients.12 Thus, this reveals that the onset of endothelial dysfunction is an important pathogenic event that precedes the development of vascular disease.
Endothelial dysfunction in congestive heart failure
The pathogenesis of heart failure is determined by the ventricular and vascular responses to myo-cellular injury. Studies have shown that the vascular endothelium may play an important role in the progression of ventricular and vascular remodeling in heart failure cases. Impaired vascular endothelium that is characterized by increased basal vasomotor tone and decreased vaso-dilatory reserve could be of particular relevance in congestive cardiac failure.13 Endothelium-dependent vasodilation has been investigated in various experimental models and clinical studies of congestive heart failure. In experimental studies on rats and dogs with induced heart failure, agonist-stimulated and flow-stimulated nitric oxide- mediated vasodilation was found to be decreased in both conduit and resistance vessels compared with normal controls.14 Similarly in clinical studies, endothelium dependent vasodilation in response to hormonal agonists and increased flow were impaired in the coronary and skeletal muscle circulations of patients with heart failure compared to normal subjects.14 Studies have also shown that endothelin-1, an endothelium-derived contracting factor is increased in both experimental subjects and clinical heart failure patients compared with normal control subjects.14 These findings demonstrate that heart failure is associated with generalized endothelial dysfunction, which is partly described by impaired nitric oxide-mediated vasodilation and increased plasma concentration of endothelin-1.
Increased peripheral vascular resistance is known to be a hallmark of congestive cardiac failure. The impaired functional capacity of peripheral blood vessels to dilate in response to sheer stress is a major determinant of the degree of exercise intolerance, which is an important clinical feature in patients with heart failure.15,16 The noted deficit in peripheral vasodilation has been attributed to the loss of ability of the endothelium to release NO in response to physiologic stimuli.17,18 Immunologic and inflammatory responses can play a role in the development of heart failure.19 Elevated levels of circulating of some cytokines, such as: interleukin-1, interleukin-6 and tumor necrosis factor-alpha, as well as certain chemokines have been observed in patients with congestive heart failure.20‒22 Such cytokines can cause direct endothelial dysfunction or indirectly through generating free radicals.23,24 Changes in regional blood flow and pressure patterns, observed commonly in patients with heart failure, may also contribute to increased free radical production25 – thus, providing a relationship between endothelial dysfunction and congestive heart failure.
Endothelial dysfunction in atherosclerosis
Either physical or subtle cellular damage to the endothelium is recognized as a significant initial event in.26,27 Physical injury to the endothelium caused by atherosclerotic lesions.28 Also, it has been shown that experimental hypertension disrupted endothelial integrity.29 Chemical injury to the endothelium caused by hyper-homocysteinemia was found to be associated with premature atherosclerosis and thrombosis.30 This finding, in association with the clinical progression of vascular disease, added support to the hypothesis of “response to injury”.31 In this context, features of injured endothelium that initiate fatty streaks and formation of plaque also include: increased adherence of monocytes and platelets, increased permeability to monocytes/macrophages and lipoproteins that accumulate in the vascular wall.32 Endothelial dysfunction has been shown to decrease the availability of local NO – either because of decreased endothelial production of NO and/ or because of excess production of superoxide anions, with consequent destruction of NO before it reaches its target tissues. As NO inhibits platelet aggregation and endothelial cell leukocyte interactions, reduced NO activity contributes to initiation and progression of atherosclerosis.33 In hypercholesterolemia, superoxide production is enhanced with consequently decreased bioavailability of NO.34 It was demonstrated that supplementation with oral L-arginine, the precursor of NO, had an antiatherogenic effect in cholesterol-fed animals35 and resulted in decreased platelet aggregation36 and reduced adhesion of monocytes to endothelial cells in humans.37 Endothelial dysfunction was featured in children and young adults who had risk factors for atherosclerosis.38 Impaired function of the endothelium was associated with hypercholesterolemia in 7year old children.39 In coronary circulation, endothelial dysfunction was present at sites of obstructive stenosis and was documented angiographically in subjects with risk factors for atherosclerosis.40,41 However, there are no longitudinal studies in humans that reveal if these young subjects with endothelial dysfunction will develop atherosclerosis. Risk factors that interact to damage the endothelium in symptomatic subjects and are involved in determining the clinical cardiovascular end points are shown in Table 2.42 Endothelial dysfunction was found to occur first at branch points of coronary arteries, which leads to occlusive arterial disease in experimental models as well as in human heart transplant recipients.43,44 Coronary microcirculation also exhibited endothelial dysfunction, which plays a significant role in the development of myocardial ischemia.45 Collectively, experimental and clinical data indicate the implication of impaired endothelial function in early and late developmental stages of atherosclerosis.
S.NO |
Conditions |
Endothelial Dysfunction |
1 |
Atherosclerosis |
Type 1 and 2 diabetes mellitus |
2 |
Hypercholesterolemia |
Hyperglycemia |
3 |
Low HDL cholesterol |
Active and passive cigarette smoking |
4 |
Hypertension |
Heart failure |
5 |
Aging |
Family history of coronary disease |
6 |
Insulin resistance |
Postmenopausal status |
Table 2 Common conditions associated with endothelial dysfunction
Assessment of endothelial dysfunction
The function of arterial endothelium has been the focus of many studies since the discovery of NO. Research in this area also involved the assessment of endothelial condition as relates to health and disease. The ability of the endothelium to produce NO under normal conditions and in response to physiologic or pharmacologic stimuli was tested in most of these studies. Assessment of endothelial functions also involved the effects of released substances on platelets, monocytes and smooth muscle cells, among many others.
Coronary artery testing
Coronary endothelial function was first assessed in vivo in humans about 30years ago46 – when the artery diameter was measured by quantitative angiography before and after intracoronary infusion of acetylcholine. It was shown that acetylcholine stimulated the endothelial release of NO and resulted in vasodilation in normal arteries. In contrast, vasoconstriction was observed in response in subjects with endothelial dysfunction. Testing of coronary microvascular endothelium by an invasive method was reported. In this technique, responses of coronary blood flow to administration of endothelium-dependent and endothelium-independent vessel-dilating substances were measured by use of Doppler wires or catheters.47 This technique has been utilized in assessing therapeutic strategies for potentials of reversibility of endothelial dysfunction in coronary arteries.48,49 The disadvantage of this intra-coronary testing is that its invasive nature would not be suitable for use in children or in adults who are at risk of atherosclerosis but without clinical signs or of the disease.
Peripheral artery testing
Detection of endothelial dysfunction in brachial and femoral arteries using a non-invasive method was first reported in 1992.38 In this technique, arterial diameter changes are monitored in response to an increase in shear stress resulting from administration of endothelium-dependent and endothelium-independent dilating agents. This technique proved to be reproducible50 and was found to correlate well with invasive testing of coronary endothelial dysfunction.51 Endothelial function was tested in the forearm microcirculation by intra-arterial infusion of endothelium-dependent and independent vasodilator substances - followed by measurement of blood flow using plethysmographic tools.52,53 Such developed methodology provided valuable insights into aspects of the atherosclerotic risk in patients and in asymptomatic subjects.
Reversibility of endothelial dysfunction
Many studies have been carried out to examine the possibility for therapeutic strategies to maintain normal endothelial functions and/or improve endothelium dysfunction. Most interventions attempting to improve endothelial dysfunction involved one or more of the known risk factors that can cause endothelial damage. For example: in cases of hypertension, ACE-inhibition was tasted; hypercholesterolemia, lipid-lowering agents were tested; cigarette smoking, cessation of smoking was tested; sedentary lifestyle, increased physical activity as a remedy was tested; menopause, hormone replacement therapy was tried; and in case of diabetes mellitus, control of associated metabolic abnormalities was tried. Collectively, several pharmacologic remedies with the ultimate objective of maintaining vascular health have been postulated. It has become known that vascular health can be ensured by having normal functioning endothelium that promotes vasodilation, inhibits vasoconstriction – among other beneficial influences.
Lipid lowering agents
It has been shown that cholesterol-lowering therapy has been associated with a decreased risk of coronary ischemic events and with an improvement in coronary endothelial function.54 Antioxidant therapy was needed to the remedy for lowering low density lipoprotein to improve vasodilation in coronary arteries,55 which emphasizes the factor of oxidative stress in the pathogenesis of endothelial dysfunction. Improvement in the vasomotor response to acetylcholine was significantly greater in combined therapies than with diet or LDL cholesterol-lowering alone.
Angiotensin-converting enzyme (ACE) inhibition
Angiotensin 2, a compound of the rennin-angiotensin system, is a potent vaso-constricting factor and in regulation of blood pressure. One of the first studies to demonstrate an improvement in endothelial dysfunction with an ACE inhibitor was the Trial on Reversing Endothelial Dysfunction (TREND).56 This trial demonstrated significant improvement in endothelial vasomotor function in normotensives patients with coronary heart disease treated with an ACE inhibitor (quinapril 40g/day). The beneficial mechanisms of quinapril in this trial probably relate to the effects of ACE inhibition on both angiotensin2 and bradykinin, which is a potent vasodilator. In the TREND study quinapril improved endothelial dysfunction without altering lipids or reducing blood pressure.56 More recently, the Heart Outcome Prevention Evaluation (HOPE) trial has shown favorable effects with ramipril in high-risk group of patients with preexisting vascular disease.57
Antioxidants
Oxidation of low-density lipoprotein cholesterol contributes to endothelial dysfunction – thus, measures to reduce this oxidative process would help in protecting the endothelium. A study has shown that vitamin C reversed endothelial dysfunction in the brachial circulation of patients with coronary artery disease.58 Epidemiological studies have shown lower incidence of cardiovascular disease was associated with diets that are rich in antioxidant content. However, clinical trials did not provide solid evidence that warrants making a recommendation for prescribing antioxidant vitamins. Thus, a sound remedy is to rely on a dietary pattern contains enough and a variety of antioxidants. The Mediterranean-type diet, for example, and similar diets would be cardiovascular-protective, as it emphasizes consumption of fruits and vegetable – among other antioxidant-containing food items.59
Hormone replacement therapy
The finding that estrogen receptors are localized on endothelial and smooth muscle cells of several mammalian species has suggested that the hormone may directly influence vascular function.60 Estrogen receptor expression has also been demonstrated in human endothelial cells suggesting, estrogen may act directly on human vascular tissue.61 Estrogen therapy has been shown to have a beneficial effect on endothelial function in postmenopausal women with atherosclerotic coronary arteries.62 This protective effect of estrogen may be due to an antioxidant effect, or an estrogen-induced enhancement of NO synthase expression. However, recommendation for hormone replacement therapy is no longer valid – but, possible alternative remedies may be tried in the future.
Other interventions
Augmentation of NO production by L-arginine supplementation has been shown to improve vascular relaxation in certain conditions.63 A recent study demonstrated improved brachial artery flow mediated dilation (FMD) in hypercholesterolemia subjects after four weeks of oral L-arginine supplementation.64 Future long term studies to explore this oral method of administration will clarify the usefulness of L-arginine in modulating endothelial dysfunction.
The endothelium is the layer of cells in the wall of blood vessels. Because it gets in direct contact with blood, several interactions and influences can result. The significance of the endothelium and the roles it plays started to be realized nearly 35years ago. Normally-functioning endothelium produces vaso-dilatory and constricting substances and others that inhibit platelet aggregation and adhesion of other circulating cells. Endothelial dysfunction has serious consequences in relation to cardiovascular disease. All measures ought to be practiced to help maintain the endothelium to function normally. A major goal of therapy for patients with cardiovascular ailments should be to improve or preserve endothelial function. Furthermore, since endothelial dysfunction occurs prior to structural vascular changes, therapy should be initiated early in patients at risk.
None.
Author declares that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.