MOJ
eISSN: 2471-139X


The aim of this work is to consider the anatomical, physiological and biochemical mechanisms of normal functional movement processes, specifically looking at the basal ganglia and its role in functional movement control. This work will be relating pathophysiological changes that may occur in the basal ganglia resulting in a movement disorder, specifically dystonia. This work will also be considering a treatment that is currently used to alleviate some of the clinical manifestations of this syndrome, namely deep brain stimulation (DBS). The objectives of this work are: to look at the physiology and biochemical mechanisms of normal functional movement processes and relate these to the basal ganglia, considering how this area regulates movement processes through neural pathways or projections; to consider the physiological and biochemical changes that DBS may have on movement disorders, specifically dystonia.
Keywords: basal ganglia, dystonia, deep brain stimulation, movement disorder
GD, generalized dystonia; FD, focal dystonia; HD, hemi dystonia; gamma-amino butyric acid; GPI, globus pallidus internus; GP, globus pallidus; PVGPI, poster ventral globus pallidus internus
Dystonia and dystonic clinical manifestations in relation to functional movement processes are very broad and indeed too broad for the objectives of this work alone, therefore dystonia will be considered as a broad spectrum neurological movement disorder for the purpose of ease of explanation. The main body of the work will explore the physiological and pathophysiological mechanisms of neural-transmission within the basal ganglia in relation to normal and abnormal functional movement, considering how pathophysiological changes alter agonist and antagonist contraction, often resulting in co-contraction as seen in many dystonic clinical manifestations.
Dystonia has been described by several authors including Berardelli et al.1 and Jiménez et al.2 as a syndrome that is clinically recognizable by prolonged muscle contractions that cause sustained twisting movements and abnormal postures of the affected body part(s). Within clinical practice, dystonia has been classified into groups depending on which anatomical area of the body is affected.3 Subgroups have been included to further gauge the type of dystonic manifestation including: focal dystonia (FD) which has been described as affecting a single body part; segmental dystonia (SD), described as affecting adjacent body parts or a segment of the body;4 hemidystonia (HD), involving one side of the body and; generalized dystonia (GD), affecting two or more segments of the entire body.5 Dystonia has also been grouped according to other underlying pathologies. Primary dystonia (PD) has been shown to occur in patients who have no signs of structural abnormality in the central nervous system, and may or may not present with the manifestation of tremor.6 Dystonia-plus (D plus) syndromes occur when the dystonic condition is combined with other underlying pathological changes.7 Secondary dystonia (SD) has been described as having an exogenous, structural or metabolic cause, while here do degenerative dystonia (HDD) has been shown to occur when there is underlying brain degeneration such as that present in Parkinson’s disease or Alzheimer's disease.8
During the process of functional movement, excitation and inhibition processes work collaboratively so that a functional movement can occur. This is achieved when agonist and antagonist muscle groups are stimulated or inhibited, creating opposing signaling to form functional movement. This occurs when contraction of the agonist muscle(s) is coordinated with relaxation of the antagonist muscle(s). This co-coordination allows for equilibrium between the agonist and antagonist muscle(s). An analogy of this may be to imagine a signal at a rail road crossing. As the barrier descends and the train passes, the traffic behind the barrier remains still until the barrier rises again thus allowing for the traffic to cross. In physiological terms, this happens as the excitation processes and inhibition processes coordinate, signaling to form functionality. The basal ganglia are the communicator to these various agonist and antagonist groups, and communicate via neural paths to form functionality. Dystonia is seen when inhibition is suppressed to the point in which co-contraction occurs, where both the agonist and antagonist muscles contract together resulting in abnormal movement.9
Researchers including Wichmann et al.,10 Alexander et al.,11 Tepper et al.12 have explored how biochemical changes in the basal ganglia control excitation and inhibition of agonist and antagonist muscle/s for neuromuscular control. It has been suggested that the basal ganglia plays a pivotal role in the balance of excitation and inhibition within functional movement control through hugely complex neural pathways, although it is still not fully understood how these pathways work. Researchers agree to some extent that dystonia causes this normal control of excitation and inhibition processes to lose equilibrium within the basal ganglia, although the actual biochemical mechanism is not yet fully understood (Figure 1).13 Currently it is not known exactly which pathway is being affected by the syndrome and whether it is the direct pathway, the indirect pathway or both that are causing this loss of equilibrium. It is believed that dystonia results from insufficient inhibition within the muscles; this may be due to the indirect pathway malfunctioning, resulting in impaired suppression of muscle activity, however this is still not fully understood.14 As mentioned earlier, when the lack of inhibition of the antagonist or surrounding muscle/s occurs, dystonic co-contraction is seen. Co-contraction is the point in which both the agonist and antagonist points are activated resulting in the muscle losing its counteractive ability as seen in normal movement. This results in flexors and extensors, for instance, firing at the same time, as opposed to counteracting each other, thus resulting in an abnormal movement. Biochemically this may be down to gamma-amino butyric acid (GABA) absorption. Several authors-including Alexander et al.,11 Packard et al.15 and Steiner et al.16 - have suggested that dystonia is caused by a shortage of the inhibitory neurotransmitter GABA. It is believed that through the loss of this inhibitory neurotransmitter normal inhibitory processes are dramatically impaired, however this is yet to be definitively proven12 Pharmaceutical therapies that have shown to benefit patients have included increasing GABA levels through medications such as benzodiazepines, gabapentin or baclofen. The aim of these pharmaceutical interventions is to elevate some of the co-contraction manifestations of dystonia.17
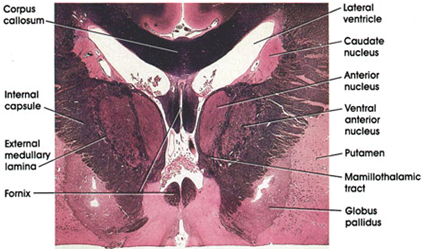
Figure 1 Weigert’s section displaying the basal ganglia in a healthy human brain Wichmann and DeLong.10
In order to consider the pathophysiological and the biochemical mechanisms that take place within dystonia, and indeed the treatment that is being considered in this work, it is important to consider the complex communications that take place in the basal ganglia in relation to functional movement control. When looking at the basal ganglia in relation to dystonia, some analogies to electrical circuitry will be used for ease of explanation of the complexity of neural pathways. The basal ganglia can be likened to a very complex circuit board with many circuitry connections. In physiological terms, this would be a complex network of neural pathways, some of which have reactive signaling and receiving properties, enabling communication and thus functionality. The basal ganglia is derived from four nuclei; these four different nuclei have a significant role in normal voluntary movement within the human body.11 The nuclei do not have direct input or output connections/pathways with the spinal cord and therefore depend on divergent paths to form functional signaling pathways in order to send movement to the various agonist and antagonist muscles. The nuclei receive their primary input from the cerebral cortex. The nuclei send their output to the brain stem via the thalamus and thence back to the prefrontal, premotor, and motor cortices.18 The motor control functions of the basal ganglia are mediated mainly by the motor areas of the frontal cortex. This mediation of motor control allows for the normal distribution of neural impulses to the many various areas responsible for functional movement.19 Research into Parkinson’s disease, Huntington’s disease, and Hemiballismus has shown pathological changes in the sub cortical nuclei, suggesting that there is a link between pathological changes in this area and movement disorder(s).18
The basal ganglia have several interconnected sub cortical nuclei with major projections to the cerebral cortex, thalamus and certain brain stem nuclei. These nuclei receive major input from the cerebral cortex and thalamus and then send their output back to the cortex (via the thalamus) to the brain stem’s sub cortical reentrant circuits, linking the cortex and thalamus.15 This system of interconnection allows for communication between the different areas and indeed control of fine signaling. This notion of signaling can be complex and overwhelming when considering neural transmissions. Dere20 talks of neural transmission and likens it to the transmitters on a circuit in which fine copper wires send and receive impulses and relay signals to the various components on the circuit board. If a disruption occurs to any of these fine copper wires, signal rate/consistency could be changed, potentially altering the functionality of the process of normal distribution of an impulse or impulses across the mass of the circuit board. This disruption could thus result in functional loss or variation in function, as seen in some dystonic manifestations. There are four principal nuclei of the basal ganglia; the striatum, the globus pallidus or pallidum, the substantia nigra (consisting of the pars reticulata and pars compacta), and the sub thalamic nucleus. The striatum has three significant subdivisions which include the caudate nucleus, the putamen, and the ventral striatum which includes the nucleus accumbens.16 The striatum is divided into the caudate nucleus and the putamen by the internal capsule, a major collection of fibers that run between the neocortex and thalamus in both directions.16 The striatum is the main receiver of inputs to the basal ganglia from the cerebral cortex, thalamus, and brain stem. The striatum’s neurons project out into the globus pallidus and substantia nigra. The globus pallidus and substantia nigra give rise to the major output projections from the basal ganglia.21 Anatomically, the globus pallidus is situated medially to the putamen and is positioned slightly laterally to the internal capsule. It is divided into an external and internal segment. The internal pallidal segment relates functionally to the pars reticulata of the substantia nigra, which lies in the midbrain on the medial side of the internal capsule.10 The cells of the internal pallidal segment and pars reticulata use amino butyric acid GABA as a neurotransmitter, which is responsible for the reduction in neuronal excitability throughout the nervous system. In relation to altered physiological processes such as the one discussed in this work, GABA absorption may become severely impaired by nerves damaged from neurodegenerative conditions and syndromes, which in turn leads to hypertonia of the muscles receiving signals from those nerves.22
In normal functionality, GABA would be distributed through the neural pathways allowing for inhibitory responses. However, when an alteration occurs – as within neurodegenerative conditions and syndromes - this normal response is interrupted, resulting in abnormal neural transitional relay.23 If imagined using the circuit board analogy, it would be as if the fine copper wires that transmit the electrical signal from point A to point B had lost some of the reactivity or conductivity, thus resulting in signals failing to travel in normal distribution patterns, culminating in functional alteration. Similarly, as the caudate nucleus is separated from the putamen by the internal capsule, the internal pallidal segment is separated from the substantia nigra. In addition to its reticular portion, the substantia nigra has a compact zone (pars compacta). The pars compacta is a distinct nucleus which lies dorsal to the pars reticulate, with some of its neurons lying within the pars reticulata. Biochemically, the cells of the pars compacta are dopaminergic and also contain neuromelanin.21
It has been suggested by several authors - including Herdegen et al.24 and Borovansky et al.25 - that neuromelanin accumulates with age in lysosomal granules in the cell bodies of dopaminergic neurons. Dopaminergic cells are also found in the ventral-tegmental area, which, anatomically, is a medial extension of the pars compacta. The effects of the increase in neuromelanin are still not completely understood, but there may be some indication that this increase negatively impacts on neural transmission(s) because the increase or accumulation may alter structure and hence functionality. However, this purely speculative. The sub thalamic nucleus is closely connected anatomically with both segments of the globus pallidus and the substantia nigra. The sub thalamic nucleus lies just below the thalamus and above the anterior portion of the substantia nigra, and the glutaminergic cells of this nucleus are the only excitatory projections of the basal ganglia.21All areas of the cortex send excitatory, glutaminergic projections to specific portions of the striatum, which also receives excitatory inputs from the intralaminar nuclei of the thalamus, dopaminergic projections from the midbrain, and serotonergic input from the raphe nuclei (Figure 2).26 The striatum itself consists of two separate parts, the matrix and striosome compartments, which have differing histochemistry as well as having different receptors. The striosome compartment receives its major input from the limbic cortex and has neural projections primarily to the substantia nigra pars compacta.27 The striatum itself contains different cell types, 90–95% of which contain GABA-ergic medium-spiny projection neurons. The cells are targets of cortical input and the only source of cortical output; and are mainly quiescent except during movement or in response to peripheral stimuli.28
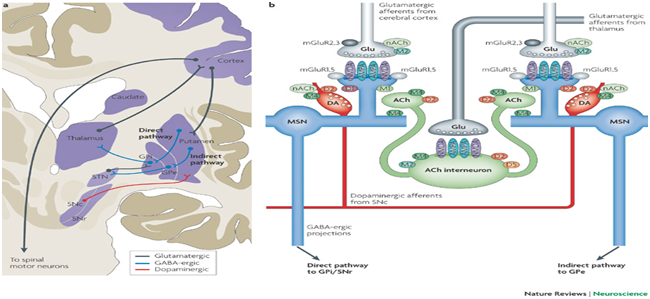
Figure 2 Diagrammatic representation of the neural pathways involved in functional movement.21
The striatum has two local inhibitory types of interneuron’s containing somatostatin, neuropeptide Y, or nitric oxide synthetase. Both of these inhibitory interneuron’s have extensive axon collaterals which reduce the activity of the striatal output neurons.12 Projections of the striatum go to output nuclei via direct and indirect pathways, the internal pallidal segment and the substantia nigra pars reticulata; and tonically inhibit their target nuclei in the thalamus and brain stem.12 The inhibitory process is believed to be modulated by two parallel pathways that run from the striatum to the two output nuclei. The two output nuclei have one direct and one indirect pathway. The indirect pathway passes first to the external pallidal segment and then onto the sub thalamic nucleus. This is in a purely GABA-ergic pathway, which continues from the sub thalamic nucleus to the output nuclei in an excitatory glutaminergic projection.29 Projection from the sub thalamic nucleus is the only excitatory intrinsic connection of the basal ganglia. Other projections from the basal ganglia are GABA-ergic and inhibitory.30 When considering normal physiological and biochemical mechanisms within the basal ganglia it is important to consider how the intervention of DBS physiologically interacts within neural pathway relay in relation to functional movement control. DBS is a surgical treatment/intervention in which fine electrodes are inserted into specific anatomical landmarks within the brain. The electrodes or stims are responsible for creating an artificial neuro-electrical stimulation to mimic normal biological/biochemical impulses.31 Anatomical landmarks for DBS include the globus pallidus (GP) area of the basal ganglia, which, as discussed earlier, is primarily responsible for inhibitory action. This target site is chosen to alleviate symptoms of over excitation processes by mimicking the inhibition of the stimulation process through pulsatile impulses from the artificial stimulation. This functionally decreases the muscular activity that causes dystonic clinical manifestations. Bain et al.,32 Tarsy et al.,33 Lee et al.34 have suggested that the basal ganglia, in particular the globus pallidus internus (GPi), may normalize levels of inhibition when stimulated so allowing for the gradual replacement of normal physiological inhibitory responses and thus clinically in reduced co-contraction manifestations. Other anatomical sites for stimulation include the thalamus, pallidum and sub thalamus. The thalamus had been used for some time as a target point for the treatment of chronic pain as well as essential tremor, because the thalamus has been shown to have outflow pathways which functionally, when stimulated, seem to have positive impacts on tremor related symptoms. Research has suggested that DBS in this region has shown significant suppression of tremor, with several studies suggesting a success rate in and around 90 %. However, research has also shown that there is sometimes no significant improvement in bradykinesia and dyskinesia.35 DBS within the poster ventral globus pallidus internus (PVGPI) has shown to adequately diminish contra lateral dyskinesia, but other dystonic types as well as non-dopaminergic gait and bulbar function have been shown not to improve with treatment, suggesting that some other neural pathways are potentially involved in these functional traits. The sub thalamus (ST) has been shown to herald the most positive outcomes for patients in relation to clinical manifestation reduction in functional movement disorder. It has been suggested that bilateral sub thalamic stimulation reduces and, in some cases, ceases symptoms of tremor, rigidity and bradykinesia. Research by Benebid36 suggested that stimulation of the sub thalamic nucleus could improve most parkinsonian symptoms, although some negative effects have been shown during electrical stimulation of the sub thalamic area, including some dystonic symptoms.
In conclusion, the complexity of neural pathways within functional movement control is undeniably complicated and far from fully understood. Movement disorders related to these neural pathways are still being explored in order to further understand how these interconnections create function/functionality, and, in turn, how pathological changes alter this. DBS as a treatment/therapy has shown that by stimulating certain anatomical areas within the brain, physiological movement mechanisms occur. Although it is becoming clearer how these areas affect different functional processes, the underlying neural pathway mapping is incomplete, thus not currently allowing for a more specific treatment/therapy option(s). Understanding of the biochemical interactions within functionality is also extremely important, as the functional mechanisms dependent on these biochemical aids in order to operate normally. Interestingly, when considering stimulation points in relation to dystonia, the GPi has been shown to benefit inhibition processes and help alleviate contraindications. The full extent of the actual mapping of the neural pathways in relation to why the GPi has this effect is still not fully understood, but does suggest that the pathways must have inhibitory actions/responses. Potentially in the future, further detailed neural pathway mapping could be performed in order to find even more specific measures to reactivate physiological functional control. Again DBS within the PVGPI has shown to have effects on contra lateral dyskinesia but not within other dystonic manifestations! But why is this and why does this happen? Theoretically, it may be that the neural pathways have been affected by pathological/biochemical changes for whatever reason, and hence these paths have altered functionally, resulting in dystonic manifestations and other clinical manifestations of movement disorder. It may also be-again theoretically speak-that neural pathways, when affected biochemically/pathologically, form divergent pathways in an attempt to continue functional communication. However, the nature of divergent pathway formation is not understood at this time, and therefore this is purely speculative. For future research and interest, neural pathway mapping for the alleviation of movement disorders would seem viable when combined with extensive biochemical analysis of inhibitory and excitatory properties of pathways. More specific areas within the brain could be targeted and analyzed to look for these inhibitory and excitatory properties. DBS for the treatment of dystonia is not currently refined enough to completely alleviate all the clinical manifestations. However, as mapping techniques improve and understanding about the interaction between neural pathways and the biochemical mechanisms within these pathways improves, further, less invasive treatment options may become available.
None.
Author declares that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.