MOJ
eISSN: 2471-139X


Histological and immunohistochemical changes in vaginal tissue structure of ovariectomized rats were assessed in response to sex steroid hormone administration. Sixty female rats were divided into six groups (10) each; control, sham, ovariectomized, ovariectomized rats treated with estradiol (20µg/kg/day), Ovariectomized rats treated with progesterone (1mg/kg/day) and ovariectomized rats treated with both estradiol and progesterone. Vaginal tissues were examined through haematoxylin and eosin, masson trichrome, and periodic acid shiff stains. Immunohistochemistry was used for expression of E-cadherin in vaginal epithelial cells. Ovariectomized rats with or without progesterone administration revealed a significant decrease in vaginal weight and length, reduction of vaginal epithelial thickness and glycogen content, which were restored with estradiol administration. Percent area of collagen fibers was not affected in all groups. E-cadherin expression was decreased in vaginal epithelium of both ovariectomized rats and progesterone administered group. It was concluded that vaginal epithelial atrophy due to ovariectomy can be restored by administration of estradiol or in combination with progesterone.
Keywords: vaginal epithelium, rat, ovariectomy, sex steroid hormones
KFMRC, king fahd medical research center; PAS, periodic acid schiff; NCL-E-Cad, novocastratm lyophilized mouse monoclonal antibody for e-cadherin; HE, haematoxylin eosin; ANOVA, one way analysis of variance; SD, standard deviation
Many women suffer from a decline of estrogen levels at menopause, which is accompanied by a number of physiological changes. Vasomotor instability (hot flushes), decreased libido, mood swings, vaginal atrophy, serum lipid changes and an increased risk of developing osteoporosis are some of the complains. Progesterone and/or estrogen therapy can improve some of these symptoms; however other women are contraindicated to take these medications due to their side effects.1,2 Steroid sex hormones are crucial to maintain the integrity of female genital tissue structure and function. Thickness and rugae of vaginal wall and its lubrication are estrogen dependent.3 In addition, estrogens can enhance genital sensation and maintain blood flow.4 Previous studies on ovariectomized animals revealed a significant reduction in vaginal epithelial height and smooth muscle tissues.5,6 E-cadherin is one of the cadherin protein groups, which is responsible for cell to cell adhesion of epithelial cells, morphogenesis, and tissue organization. Impairment of E-cadherin expression is correlated with tumor invasion and metastasis in gastric and ovarian cancers, and some other tumors.7,8 Estrogen was as key regulators of E-cadherin expression and growth in adult female reproductive tract. Estradiol has been known as a potent stimulator of E-cadherin expression in mice ovary9 and uterus.10
This study aimed to explore the possible role of sex steroid hormones to restore histological changes in ovariectomized rat vagina.
Animals
Sixty female albino Wister rats (8weeks old and weigh 225-285g) were purchased from the animal house of King Fahd Medical Research Center (KFMRC) - Jeddah, Saudi Arabia. Animals were maintained in the same facility in accordance with the guidelines and ethical rules of the Canadian Council on Animal Care. Rats were maintained at a constant temperature of 22-24°C and 55% humidity, with a 12hr light/dark cycle. They were allowed free access to food and water (ad libitum) and were acclimatized for one week before starting the experiment.
Experimental design
After acclimatization, animals were randomly divided into six groups (10 rats each).
Group I: (-Ve control): no surgical operation.
Group II: (+Ve control): Animals underwent sham operation where rats were anesthetized by ether inhalation, followed by antiseptic cleaning of their skins. Small surgical incisions were made in the lower midline before suturing it back.11 All the rest of 40 rats were anesthetized, cleaned with antiseptic and subjected to lower midline incision as in Group II. Ovaries were removed after tying off and cut from the oviduct. Local antibiotics were applied before suturing the muscles and the skin to close the incision.12 After operation, rats were randomly distributed into 4 groups (10 each)
Group III: (OVX+S): Ovariectomized rats received saline via intragastric tube and were sacrificed 4weeks after ovariectomy.
Group IV: (OVX+E): Ovariectomized rats received estrogen in the form of oestradiol benzoate (Folone ampoules - Misr Company), which was injected subcutaneously for 4weeks with a dose of 20µg/kg/day.13
Group V: (OVX+P): Animals received progesterone in the form of norethisterone acetate (steronate - Hi Pharm Company), which was given orally by gastric tube for 4weeks at a dose of 1mg/kg/day.14 Drug was prepared by grinding 5mg tablet into a powder that was suspended in 5ml distilled water.
Group VI: (OVX+E&P): Rats were given both estrogen and progesterone in the above regimen for 4weeks.
After 4weeks rats were weighted, anaesthetized with Ketamine then sacrificed by cervical dislocation. Vaginas were excised, weighted and lengths were measured. The middle portions of vagina were fixed in 10% buffered formalin, divided longitudinally after 48hrs and prepared for paraffin embedding.
Histopathology
Vaginal sections of 4µm in thickness were stained with haematoxylin and eosin (H&E).15 Periodic acid Schiff (PAS) reaction and Masson trichrome stain were used to detect glycogen and elastic fibers in vaginal sections, respectively.15
Epithelial thickness
Vaginal epithelial thickness was performed in slides stained with H&E. Four images were taken for each slide representing an animal. Ten measurements were used for each image and the average epithelial thickness was calculated. Images of histological sections were taken under a final magnification of x400 and analyzed using Image-Pro Plus 4.5 software (Media Cybernetics, Silver Spring, MD,USA).16
Percent area of collagen fibers
Eight images for each slide of vaginal sections stained with Masson trichrome were used and a 100-point grid mask was applied to count the average per cent area of collagen fibers per group.16
Immuno histochemistry for E-cadherin
Sections were mounted on poly-l-lysine-coated slides. NovocastraTM Lyophilized mouse monoclonal antibody for E-cadherin (NCL-E-Cad) was used in 1:50 dilution in an avidin-biotin detection system, following the manufacturer's instructions (Ventana Bench Mark XT, Ventana Inc., Tucson, AZ). The immunohistochemical procedure was carried out by using an automatic immunostainer. The reaction was visualized with the standard 3, 3 diaminobenzidine (DAB). Sections were counterstained with haematoxylin to facilitate detection brown color of E-cadherin expression17 and examined then photographed using a light microscope (BX51, Olympus Optical Corporation, Tokyo, Japan) fitted with an Olympus digital camera (DP20).
Statistical analysis
Results are provided as means and standard deviation (SD). One way analysis of variance (ANOVA) was used to test the significant difference between the six groups. IBM® SPSS 19 Statistics Software Inc. was used for analysis of the obtained data and results were considered statistically significant if P<0.05.
Influence of ovariectomy and hormone replacement on body and vaginal weight and length
Body weight increased in all groups during the experiment period (4weeks). There was no significant difference in body weight between all rat groups involved in the experiment. Vaginal weight and length decreased significantly (P<0.05) in OVX+S and OVX+P groups compared to the rest of rat groups, (Table 1).
Groups |
Body weight (G) |
Vaginal weight (G) |
Vaginal length(Mm) |
|
Start |
End |
|||
-Ve Control |
220±4.5 |
350±14.1 |
0.25±0.010 |
25.0±0.01 |
+Ve Control |
210±4.4 |
346±13.8 |
0.24±0.011 |
24.0±0.01 |
OVX+S |
215±4.1 |
348±13.9 |
0.19±0.010* |
18.8±0.04* |
OVX+E |
218±4.3 |
349±13.9 |
0.25±0.012 |
24.1±0.03 |
OVX+P |
200±4.4 |
340±13.0 |
0.20±0.010* |
18.0±0.02* |
OVX+E & P |
205±4.2 |
345±13.7 |
0.23 ±0.012 |
23.9±0.05 |
Table 1 Effect of ovariectomy and hormone treatment on body and vaginal weight and length
*Significant differences between groups were assessed using one way ANOVA P<0.05
Influence of ovariectomy and hormone replacement on the vaginal epithelium
Both negative and positive control groups had stratified squamous epithelium of six to eight cell-layers thick. Basal layer cells contained large round nuclei. Intermediate zone cells were more flattened and the long axis of the nuclei was parallel to the basement membrane and epithelial surface. Nuclei were darker and condensed than those of cells in other layers (Figure 1). Vaginal epithelium thicknesses in negative and positive control animals were (72.5±5.1µm) and (70.1±4.8µm), respectively (Figure 2). Fourweeks after ovariectomy, vaginal epithelium layers were decreased to two or three layer thick in OVX+S group. Morphometric analysis of epithelial cell thickness was (30.6±1.2µm) which, confirmed a significant decrease (P<0.05) compared to all other groups (Figure 1) (Figure 2). Cells had more condensed nuclei and less surrounding cytoplasm. Basal cells were cuboidal with nearly squared nuclei compared to round or oval nuclei that were present in the control groups. Estradiol administration dosage of 20µg/kg/day for 4weeks restored the thickness of the vaginal epithelium (69.1±3.9µm) after ovariectomy. An obvious thickening of vaginal epithelium in OVX+E animals was apparent and contained approximately 10-12 hyperplasic cell layers (Figure 1). Basal epithelial cells were packed tightly and similar to those in the controls. The more superficial layers had gradual flattened cells. OVX+P group that received a dose of 1mg/kg/day for 4weeks had vaginal epithelial proliferation (66.3±3.8µm) but was not similar to negative control (72.5±5.1µm) or to positive control (70.1±4.8µm) animals (Figure 1) (Figure 2). Co-administration of estradiol (20µg/kg/day) and progesterone (1mg/kg/day) to ovariectomized rats OVX+E&P sustained vaginal epithelium thickness (69.5±3.7µm) close to those observed in control groups (Figure 1) (Figure 2). Basal cells in this group were columnar with vertically oriented oval nuclei. Cells were flattened toward the luminal surface with more squamous nuclei. There was only sparse epithelial enfolding into the lamina propria similar to those in control animals.

Figure 1 Vaginal epithelium in –ve control, +ve control, OVX+E, and OVX+E&P rat groups consisted of six to eight layers of cells ({) with apparent basal cell layers in –ve control group (arrows). OVX+S rats had obvious vaginal atrophy characterized by reduction in epithelial layers ({). (OVX+P) group had no increase in vaginal epithelium thickness nor did it reach control levels. Cornfield epithelial cells (*) remained in the vaginal lumen of OVX+S and OVX+E&P groups. Scale bar=50µm, H&E stain
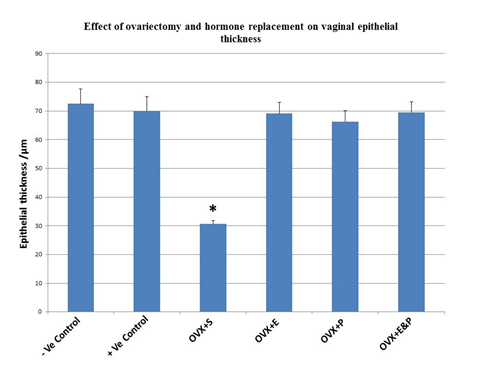
Figure 2 Histogram showing significant decrease in vaginal epithelial thickness (*P<0.05) in Ovariectomized OVX+S rats compared to all other groups. Data represented as means±S.D. Significant difference between groups were assessed using one way ANOVA P<0.05.
Influence of ovariectomy and hormone replacement on the muscularis layers
Muscularis layers in both negative and positive control groups were well defined consisting of circular and longitudinal smooth muscle fibers. Individual muscle fibers were arranged in bundles, which were enclosed within thin connective tissue septa (Figure 3). No differences were noticed in the structure of muscularis layers in OVX+S, OVX+E, OVX+P, or OVX+E&P rat groups when compared to the -ve control and +ve control rat groups (Figure 3).
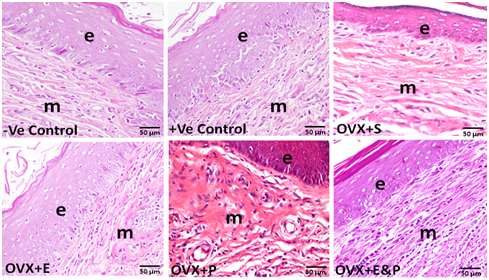
Figure 3 Muscularis layers (m) and vaginal epithelium (e) in all six groups. No structural changes were found in the muscularis layers (m) in all six rat groups. Scale bar=50µm, H&E stain.
Collagen and elastic fibers detection in vaginal sections
Morphology of the collagen and elastic fibers in vaginal sections were not affected in OVX+S, OVX+E, OVX+P, and OVX+E&P rat groups when compared to negative and positive control groups (Figure 4). These findings were confirmed by histomorphometry, which revealed that percent area of vaginal collagen fibers to be (70±1.2) in -ve control; (68±1.1) in +ve control; (67±1.03) in OVX+S; (69±1.09) in OVX+E; (66±1.02) in OVX+P; and (67±1.04) in OVX+E&P. No significant difference was noticed between control groups and all other rat groups in the percent area of vaginal collagen fibers (Figure 5).
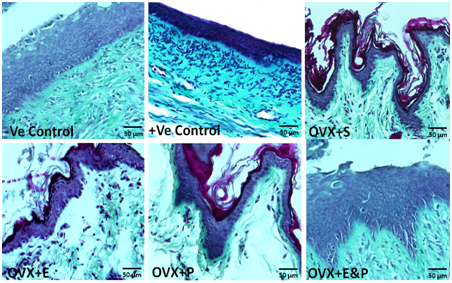
Figure 4 Morphology of vaginal collagen and elastic fibers was not different in all six groups. Scale bar=50µm, Masson trachome stain.

Figure 5 Histogram shows that neither ovariectomy in OVX+S group nor hormonal replacement therapy in OVX+E, OVX+P, and OVX+E&P rat groups had significant effect on percent area of vaginal collagen fibers compared to control groups. Data represented as means±S.D. Significant difference between groups were assessed using one way ANOVA P<0.05.
Glycogen detection
Periodic acid Schiff (PAS) reaction was used to detect glycogen presence in vaginal sections in all rat groups. Glycogen was observed in negative and positive controls, OVX+E, and OVX+E&P rat groups but was deficient in OVX+S and OVX+P rat groups. PAS-positive reaction was seen in superficial epithelial cells with keratohyalin granules (Figure 6).
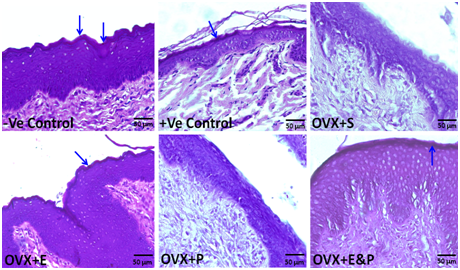
Figure 6 Glycogen detection in superficial epithelial cells with keratohyalin granules (arrows) of -ve and +ve controls, OVX+E and OVX+E&P rat groups by using PAS-positive reaction. However, it was deficient in OVX+S, and OVX+P rat groups. Scale bar=50µm, PAS stain.
E-cadherin expression in vaginal epithelium
Visualization of E-cadherin on the epithelial membrane and cell borders permitted a clear picture of the shape of the epithelial cells. Expression of E-cadherin on the lateral surfaces of the epithelial cells was lower in ovariectomized rats OVX+S and in ovariectomized rats with progesterone OVX+P in comparison with negative control and positive control groups. Treatment of ovariectomized rats with estradiol (20µg/kg/day) alone OVX+E or in combination with progesterone (1mg/kg/day) OVX+E&P increased E-cadherin expression in vaginal epithelial cells to resemble those in control groups (Figure 7).
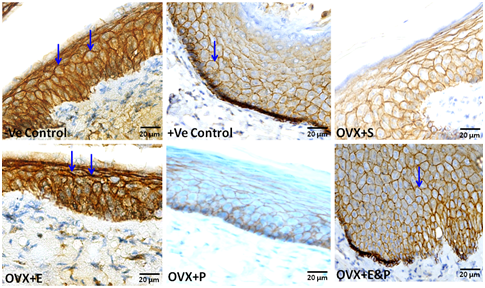
Figure 7 E-cadherin immunoreactive expression confined to the epithelial compartment. Expression of E-cadherin on the lateral surfaces of epithelial cells characterized -ve and +ve control groups, OVX+E and OVX+E&P rat groups (arrows). Lower E-cadherin expression distinguished vaginal epithelium of OVX+S and OVX+P rat groups. Scale bar=20µm, E-cadherin immuno stain.
Menopause is a special phase in women’s lives that is associated with health changes and diseases. Hormone replacement therapy is crucial to improve the quality of life and to prevent medical complications following menopausal phase. Both menopause and hormone therapy have their effects on vaginal epithelial cells. Ovarian steroids deficiency due to surgical and/or natural menopause initiates morphological and structural changes in the vagina.18,19 Forsberg18 reported that as women advance in their age vagina became short, narrow and lose their folds; moreover, the epithelial surface became flattened and keratinized. Menopause was responsible for inducing a reduction in the number of vaginal epithelial layers, and loss of intermediate cells, represented by an overall reduction in epithelial height.20 Laboratory studies, showed that ovariectomized rabbits, rats, and nonhuman primates had significant decrease in vaginal epithelial height and smooth muscle tissues.5,6,19,21
In the present study, we explored the possibility to ameliorate vaginal atrophy in ovariectomized rats by administration of the sex hormones, estrogen and progesterone. A significant decrease in vaginal weight and length were noticed in ovariectomized rats OVX+S and those that were given progesterone (1mg/kg/day) for 4weeks OVX+P compared to the other groups (negative and positive control, OVX+E, and OVX+E&P. Body weight in all six groups had no significant difference. The Low estrogen levels intensified vaginal mucosa atrophy as noticed by Sato T et al.,5 Buchanan DL et al.22 and while we did not directly quantify these changes, diminished mucosal content is likely to account for the bulk reduction in vaginal mass of OVX rats. These findings were in accordance with those established in a previous study.23
Ovariectomy was responsible for the significant decline in vaginal epithelium thickness and glycogen content. The decrease in vaginal epithelium layers rendered it susceptible to abrasion. Previous studies in animals16,24,25 and human studies26 were in agreement with our findings. The decrease in vaginal epithelium thickness would make it vulnerable to bacterial infection27 and more easily to be torn. The decrease in glycogen production would not maintain the low pH level needed in the vaginal fluA non significant muscular atrophy was confirmed in the vaginal muscularis structure of all ovariectomized and both control rat groups resembled results established by previous study.24 However, some earlier reports showed significant greater atrophy in the muscularis layers after ovariectomy in rabbits and menopause in women, respectively.21,28 This disagreement may be attributed to the relatively short term after ovariectomy during the present study (4weeks), which did not allow significant changes in the muscularis layers to emerge in ovariectomized animals. Administration of estradiol (20µg/kg/day) alone or in combination with progesterone (1mg/kg/day) for 4weeks to OVX rats maintained the structure of vaginal tissue; vaginal weight, and length; vaginal thickness and glycogen content unchanged. Results from previous studies were similar and supported data obtained in the current study.16,24 However, administration of progesterone (1mg/kg/day) alone for 4weeks to OVX rats in the present study failed to retain the structure of vaginal tissues from degradation induced by ovariectomy. Quantitative measurements of epithelial thickness in OVX+P rats were not statistically different from control rat groups, which agree with those in previous study.24
Estradiol treatment (20µg/kg/day) alone or co-administration with progesterone (1mg/kg/day) for 4weeks had no effect on percent area of vaginal collagen fibers in OVX rats. Similar findings were reported by previous study.16 However a divisive study29 showed that estrogen treatment in women decreased collagen content compared to placebo treatment. Estradiol administration in diabetic mouse significantly increased the volume of the muscle layer and the lamina propria, but induced less marked changes in elastic fibers.30 E-cadherin expression in vaginal epithelium was decreased in OVX and OVX+P rats compared to other rat groups during the current study. A previous experiment31 pointed out that progesterone receptor B mediated the decrease in E-cadherin protein expression due to epithelium abrasion and tumor invasion. Studies to female mice genital organs demonstrated that estradiol can stimulate E-cadherin expression.9,10 This might explain the strong expression of E-cadherin in vaginal epithelium in control and estrogen administered OVX rat groups.
Ovariectomy has detrimental role on vaginal structure and morphology. This was characterized by a decrease in vaginal weight, length, epithelial thickness, glycogen content and E-cadherin expression. Estradiol administration in a dose (20µg/kg/day) alone or with progesterone dose of (1mg/kg/day) for 4weeks could maintain the integrity and/or restore changes in ovariectomized rats. Administration of progesterone alone (1mg/kg/day) to OVX rats failed to restore vaginal histological changes induced by ovariectomy.
None.
Author declares that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.