Journal of
eISSN: 2475-5540


Research Article Volume 1 Issue 7
1University of Washington, USA
2Seattle Biomedical Research Institute, USA
3AVI-Biopharma, USA
4NCI, Cancer and inflammation Program Center, USA
Correspondence: Stephen Bartelmez PhD, BetaStem Therapeutics Inc, 2 Lower Crescent Ave, Suite 2, Sausalito CA 94965, USA, Tel 415-887-9820
Received: October 31, 2016 | Published: December 20, 2016
Citation: Bartelmez SH, Storey C, Iversen P, et al. Transient inhibition of endogenous transforming growth factor- β1 (tgfβ1) in hematopoietic stem cells accelerates engraftment and enhances multi-lineage repopulating efficiency. J Stem Cell Res Ther. 2016;1(7):258-267. DOI: 10.15406/jsrt.2016.01.00045
We show that ex vivo transient inhibition of endogenous transforming growth factor-β1 (TGFβ1) in highly enriched and partially enriched murine hematopoietic stem cells (HSC): (1) accelerates engraftment of long-term repopulating HSC ("LTR-HSC"), (2) increases donor stem cell chimeras in competitive bone marrow repopulation studies, (3) permits transplant of as few as sixty LTR-HSC to rescue mice from hematopoietic death after lethal irradiation using direct (non-competitive) transplants and (4) promotes extended survival in vitro of single or multiple LTR-HSC in the absence of growth factors. Our approach to inhibit TGFβ1 involved use of either neutralizing monoclonal antibodies ("TGFβ-MAB") or antisense phosphorodiamidate morpholino oligomers ("TGFβ1-PMO") prior to iv transplant. Our previous studies showed that TGFβ1 is a potent reversible inhibitor of LTR-HSC proliferation. Unexpectedly, LTR-HSC treated with TGFβ-MAB and transplanted 1-2hr later, or treated with TGFβ1-PMO for 16hr then transplanted, rapidly engrafted and produced relatively high levels of donor chimeras that persisted for >6-10 months. Early post-transplant, donor neutrophils predominated but only if TGFβ1 was inhibited in the LTR-HSC. Finally, we tested the ability of LTR-HSC to rescue mice from hematopoietic death after lethal irradiation: as few as 250 LTR-HSC treated with TGFβ-MAB or TGFβ1-PMO rescued essentially 100% of mice and produced a durably graft. In contrast, control treated LTR-HSC (untreated, isotype control MAB, or control-PMO) essentially could not rescue lethally irradiate mice. In cases where donor stem cell numbers are limiting, these methods could prove to be clinically useful. Our more recent studies have demonstrated that human specific TGF-β1-PMO can reverse the dysfunctions of diabetic lin -CD34+CD45+ stem cells to be able to repair endothelium in the retina.
Keywords: hematopoietic stem cells, hsc transplantation, tgfβ1, pmo
HSC, hematopoietic stem cells; LTR-HSC, long term repopulating-hematopoietic stem cells; STR-HSC, short term repopulating-hematopoietic stem cells; MAB, monoclonal antibodies; PMO, antisense phosphorodiamidate morpholino oligomers; Hö, höechst 33342; Rh, rhodamine 123
HSC transplantation efficiency can be improved by modulating cell surface molecules such as CD26 and CXCR4.1,2 This is important in cases where donor cell numbers are limiting, as in cord blood stem cell transplantation in adults where high morbidity and slow engraftment occur3 due to limiting numbers of CD34+ cells and possibly more differentiate progeny.4 The approach involving ex vivo expansion of primitive HSC to increase the total transplantable cell number has not been clinically useful.5,6 Here, we took the approach of enhancing HSC engraftment without expansion by transiently inhibiting endogenous TGFβ1 expression in HSC using minimal ex vivo manipulation. We previously reported that TGFβ1 directly and reversibly inhibits the initial cell divisions of murine LTR-HSC as well hematopoietic progenitor cells in vitro.7,8 TGFβ1 has been shown to be a primary regulator of LTR-HSC quiescence (G0) in bone marrow niches.9 Adult mice with a conditional knock-out of the TGFβ1 receptor exhibited essentially normal in vivo hematopoiesis.10 However, HSC/progenitor cells released from G0 quiescence in vitro by TGFβ neutralizing antibodies resulted in improved retroviral gene transfer.11,12 Furthermore, inhibiting Smad signaling, the intracellular regulators of TGFβ1 signaling, promoted HSC self-renewal in vivo.13 Here we studied highly enriched murine LTR- HSC from lineage-negative bone marrow cells followed by FACS sequential selection of low retention of the fluorescent, viable dyes Höechst 33342 (Hö) (binding A-T base pairs of DNA) and Rhodamine 123 (Rh) (binding predominately activated mitochondria membranes). Low retention of the dyes is due to both high efflux rates and low target binding. Our previous studies showed that selecting cells that are lin-, Hö low (~G0),14 c-kit+7,15 identifies both LTRHSC and short -term repopulating (STR-HSC). A further selection step based on Rh fluorescence resolves the LTR-HSC (low Rh) and STR-HSC (high Rh). LTR-HSC are predominantly quiescent9 compared to STR-HSC, and are unique in their long-term repopulating ability due a high probability of self-replication while at the same time generating large numbers of STR-HSC16 Untreated LTR-HSC have poor short-term repopulating ability early after transplant, however over time in vivo give rise to daughter cells that possess efficient short-term repopulating ability. We show that transient inhibition of endogenous TGFβ1 in LTR-HSC ex vivo by PMO or anti-TGFβ1- AB (1D11.16) significantly increases their ability to engraft murine bone marrow.
Animals
Three- to six-month-old male congenic B6SJL CD45.1 and C57BL/6J CD45.2 mice were purchased from Jackson Laboratories (Bar Harbor ME) and housed in an IRB approved facility at Seattle Biomedical Research Institute, Seattle, WA.
Growth factors and antibodies
Purified, recombinant growth factors were generously provided by and used as follows: rat SCF (50 ng/ml) from Dr. Krisztina Zsebo (Amgen Inc., Thousand Oaks CA); murine IL-3 (10 ng/ml) from Dr. Andrew Hapel, (Australian National University); human IL-6 (10ng/ml) from Dr. Douglas Williams (Immunex Corp., Seattle WA); TGFβ monoclonal antibodies (MAB) 1D11.16, 2G1.12 and 2C7.14 were generously provided by Jim Dasch (Celltrix Corp, Santa Cruz, CA), while the and 2G7 were kindly provided by Mike Palladino (Genentech Corp, San Francisco, CA). IgG1K isotype control antibodies were purchased from R&D systems (Minneapolis MN). Fab2’ fragments of 1D11.16 were generously provided by Bruce Blazar.17
Enrichment for LTR-and STR- HSC: pre-fractionation of bone marrow
Mice were euthanized, femurs and tibias were removed aseptically, and marrow was harvested by flushing with phosphate buffered saline containing 2% fetal bovine serum (PBS/2% FBS). Low-density cells were first isolated on a gradient by layering 5 ml aliquots of 107unfractionated cells/ml over 4.0ml Nycodenz (1.080g/ml, Nycomed, Norway). Cells were centrifuged 20 minutes at x 400g, collected from the interface and washed with a 10-fold excess of PBS/2% FBS. Lineage committed cells were then removed by the following antibody-mediated magnetic bead depletion method. A double strength mixture of rat antimouse monoclonal antibodies (denoted Ab mix) was prepared as follows: anti-7/4, 4% vol./vol. (antigen expressed by neutrophils and activated macrophages, a gift from Professor Simon Gordon, Oxford, UK); anti-YW25.12.7, 10% vol./vol. (blasts and nucleated erythroid cells, a gift from Dr. Ivan Bertoncello, Peter MacCallum Cancer Institute, Melbourne, Australia); antiTer119, 4ug/ml (nucleated erythroid cells); anti-B220, 9ug/ml (B and pre-B lymphocytes); anti- CD4 and -CD8, 5ug/ml (T lymphocytes); antiCD11b (Mac-1), 9ug/ml (granulocytes, macrophages); and anti-Gr-1, 11ug/ml (granulocytes, monocytes). The latter six antibodies were purchased from PharMingen (San Diego CA). Briefly, the light density cells (<1.080g/cc) were resuspended in PBS/2%FBS at 10x106 cells/100ul of PBS, an equal volume of double strength Ab mix was added, and cells were incubated at 4˚C for 15 minutes. Cells were centrifuged through 2ml of FBS to separate cells from unbound antibodies, and were then transferred into 50ml centrifuge tubes (Corning, Corning NY) and rosetted with magnetic beads coated with sheep anti-rat IgG (Dynabeads M-450, Dynal, Inc., Great Neck NJ) using the following procedure: 10 times the number of magnetic beads in an equal volume was added dropwise to the cell suspension adjusted 350x106 cells/3.5 ml maximum per tube and mixed gently with a 5-ml pipette. The suspension was centrifuged at 20g for three minutes, then vigorously resuspended and transferred to a 15ml polypropylene tube. Then 4ml of PBS/10% FBS was added to reduce non-specific cell trapping into bead-cell aggregates, and the tube was placed into the Dynal magnet for three minutes. Cells not bound with the magnetic beads remained in suspension and were carefully removed with a pipette (and designated lineage negative, “lin-“). Lin- cells were then counted, centrifuged and re-suspended in 10uM Höechst 33342 (Hö), Sigmal, St Louis MO) in PBS with 10% FBS (adjusted to pH 7.2 with NaHCO3) and incubated at 37˚C for one hour. After 40 minutes of Hö incubation, Rhodamine 123 (Rh, Sigma) was added to the cell suspension (final concentration 0.1 ug/ml; i.e., 20 minutes total incubation with Rh). After dye incubation, cells were centrifuged, chilled to 4˚C, washed once with PBS/2% FBS, labeled with phycoerythrin-conjugated anti-c-kit antibody (2B8 clone, PharMingen; 1 ug/ml final concentration) for 10 minutes, then washed and re-suspended at 0.75x106 cells/ml in PBS/2% FBS. Finally, propidium iodide (Sigma) was added at 2ug/ml for detection of dead cells.
Enrichment for LTR-HSC and STR- HSC: fluorescence activated cell sorting
The above pre-fractionated cells were analyzed and sorted on a FACStar Plus flow cytometer (Becton Dickinson, San Jose CA) equipped with dual argon lasers, and an automated cell delivery unit (ACDU). Cells were kept chilled at 4˚C with a recirculation water bath. Monochromatic light at 351-364nm and 488nm was used for Hö and Rh excitations, respectively. Forward light scatter was detected using 488bp10 and ND 1.0 filters. Hö emission was detected using a 515-long pass filter in order to maximize signals from hematopoietic stem cells. Rh emission was detected using a 530 bandpass20 filter, PE emission using a 575bandpass 20 filter, and PI emission using a 610-long pass filter. Cells were gated as follows: first, forward light scatter and PI fluorescence were analyzed, and viable cells (PI negative) were selected. Cells in these gates were further refined by selecting specific percentages from the Rh fluorescence histogram: the lowest 10% (defined as Rh low) and the middle 40% of the peak (defined as Rh high).1 Then Rh low and Rh high cells were analyzed for Ho fluorescence and c-kit receptor. Cells that simultaneously demonstrated low Ho fluorescence and expressed c-kit receptor were sorted as individual cells into 96-well plates or collected in bulk. These sorted fractions were defined as: linHölowc-kit+Rhlow and lin-Hölowc-kit Rhhigh, respectively, henceforth designated “LTR-HSC” and “STR-HSC”.
Culture conditions
Single and multiple sorted cells were cultured in 96- well U-bottomed plates (Corning) in “Medium”: IMDM medium (Gibco BRL, Grand Island NY) with 10% horse serum (HS, Gibco), 10 fetal bovine serum (FBS, Gibco), 2x10-5 M 2-mercaptoethanol (2-ME, Sigma), 10-7 M hydrocortisone (HC, Sigma) and antibiotics (penicillin/streptomycin, Gibco) supplemented with TGFβ-MAB, TGFβ1-PMO and cytokines as indicated.2 Different lots of FBS and HS used in cultures were previously screened for their ability to support HPP colony formation of lin- cells in agar. Serum-free medium formulated for hematopoietic cell support (QBSF-58, (Quality Biological, Gaithersburg, MD) contains neither 2-ME nor HC. In all experiments, cells were cultured for 1 hour -21 days with and without antibodies and PMOs as indicated in the Results. Multiple cells per well and single cells per well were verified for cell numbers by direct observation at 200X magnification at the center-bottom. Up to ~64 cells per clone could be counted accurately. At 14 days of culture the clone size was determined by counting using a hemocytometer. Where indicated, single cell survival was determined by addition of SCF (50 ng/ml), IL-6 (10 ng /ml) and IL-3 (10 ng/ml) to form colonies from single cells and colony morphology was determined on cytospins stained with Wright-Giemsa.
Long term in vivo repopulation assay
Competitive repopulation or direct transplantation (no competitor/ support cells) was used to measure repopulating ability of LTR-HSC and STR-HSC. LTR-HSC cells purified from CD45.1 mice (B6.SJL- Ptprca Pep3b/BoyJ (Ly5.1), B6.SJL) were deposited directly from the sorter into 96- well plates containing 100ul “medium” containing 20ug/ml TGFβ-MAB (ID.11.16), 20ug/ml isotype control antibody (IgG1K), 40 ug/ml TGFβ1-PMO or 40ug/ml Scrambled-PMO. Competitive or direct repopulation assays were performed using CD45.1/45.2 congenic mice. Recipient animals (C57BL/6J CD45.2) were exposed to a single dose 950cGy total body irradiation and 2-4 x105 unfractionated (CD45.2) bone marrow cells were added to wells containing CD45.1 LTR-HSC donor cells and injected iv into the tail vein of the recipient. Three weeks to 12 months after transplantation the proportion of donor-derived (CD45.1) nucleated leukocytes in the recipient's peripheral blood was quantitated by FACS analysis. Peripheral blood (100 µl) was obtained by capillary puncture of the orbital venous plexus and transferred into 1ml PBS/2% FBS, centrifuged for five minutes at x400g, resuspended in 100ul of PBS/2%FBS, and red blood cells were lysed with 1 ml of NH4Cl lysis buffer for 10 minutes at 37˚C. Then 2ml of PBS/2% FBS was added; cells were centrifuged for 10 minutes at x400g and washed twice with PBS/2%FBS. The nucleated cells were divided into two fractions and stained with fluorochrome-conjugated monoclonal antibodies specific against either CD45.1 antigen (A20 clone) or CD45.2 antigen (104 clone, PharMingen). After staining, cells were analyzed on a BD Facscan or Epics Profile II (Coulter Electronics, Hialeah FL). Red cell contamination was eliminated by analyzing only CD45.1 and CD45.2 positive cells. Nonspecific binding of anti-CD45.1 antibody was determined by control binding to CD45.2 leukocytes.
Intracellular TGFβ1 detection by flow cytometry
For detection of endogenous TGFβ1 we used Lin-Sca-1+, c-kit+ sorted cells in order to obtain sufficient cell numbers for this approach. However, ~90% of the HSC in this cell preparation are STR-HSC, with the remaining HSC being LTR-HSC (Bartelmez, unpublished data). Cells were cultured overnight. For intracellular staining, half the cells were unstimulated at a million per 1 ml culture medium in 24-well plates. The other half of cells was stimulated at 37˚C for 6 hours with 50µl PMA (diluted 1:1000 from 1mg/ml stock), 5µl ionomycin (of 1:10 dilution of 2 mM stock) and 5µl monensin (of 1:10 dilution of 2 mM stock). Cells were then washed once with PBS and once with 2% Human AB sera/0.25% Saponin/0.01% Sodium Azide/PBS (permeabilization buffer) then re-suspended in 100µl of permeabilization buffer and 2µl of mouse sera. After incubating at 4 degrees for 15 minutes, the cells were washed once and resuspended in 100µl permeabilization buffer. 15µl of PE-labeled mouse anti-human labeled TGFβ1 antibodies developed for intracellular detection (IQ Products, Groningen, Holland), and anti-actin (PharMingen) as a permeabilization control. The cell antibody mixture was incubated a 4˚C for 30 minutes. After incubation, the cells were washed twice in permeabilization buffer, to remove any unbound antibody, and then re-suspended in 0.5 ml 1% paraformaldhyde/PBS for analysis. Cells were analyzed on a FAC Scan flow cytometer (Becton Dickinson) was analyzed using Flowjo software (Treestar, Palo Alto, CA).
PMO structure and intracellular half-life
PMOs were synthesized at AVI-BioPhama (Corvallis, OR) [18,19]. We have looked at the PMO half-life in activated and non-activated T-cells and find it to be 2-4 days. The best predictor of residence time of a PMO in a cell is that it is proportional to the amount of target RNA in the cell. Neutral PMOs can enter HSC but the efflux signal is the PMO/RNA duplex which carries a significant negative charge from the RNA that remains bound even when the flanking/non-duplexed RNA is degraded. We found PMO/RNA duplexes in the urine of animals treated with PMOs (data not shown). Low numbers of PMOs will saturate the binding of cellular RNA (~200 copies per cell for RNA). However, estimates of PMO copy number per cell run much higher in the range of 100,000 copies per cell. PMO concentrations were 40µg/ml at day 0 (optimal concentration based on previous dose response studies, data not shown). Optimal nucleotide sequences were determined from murine mRNA TGFβ1 sequences and empirically testing in both in vitro and in vivo assays. The control PMO sequence was 5’ CGT TCT GAT AGC TGT ACC TC 3’ (AVI BioPharma Sequence ID# 0-1-0-555) while the TGFβ1-PMO sequence was 5’ GAC GGC GGC ATG III GAG GC 3’ (AVI BioPharma sequence ID# 0- 1-1-1067). Lyophilized control or TGFβ1-PMOs were diluted in IMDM to produce a stock solution of 300mM. HSCs were incubated overnight (16hr) at 37˚C in IMDM containing 10% horse serum, 10% fetal calf serum, 100 units/ml of penicillin, 10µg/ml streptomycin, 20mM l-glutamine and 10-6 M hydrocortisone. This incubation period was determined by measuring the uptake of FITC-labeled PMOs in murine LTR-HSC (data not shown).
Preparation and use of pAdeno (TGFβ1) GFP AT
(TGFβ1) GFP A T was obtained by PCR of pCiJNeo (TGFβ1) GFP A T with primers PCNNhe890 and PCN 1167rev. This fragment containing a chimeric intron of PCINeo, a TGFβ responsive element and GFP minus the start codon was subcloned into pShuttle vector at NheI and NotI sites. A Ceu/Ste fragment was then transferred to adeno X DNA according to the manufacturer’s instructions (Clonetech) to form pAdeno (TGF-β) GFP A T. Adenovirus was added to HEK-293T cells plated overnight at 5x104 cells/well in 0.5ml DMEM +10% FBS in a 24-well plate. Cells were harvested 3 days after culture and lysed by 5 freeze/thaw cycles. The virus preparation was centrifuged for 10 minutes at 2000xg in a refrigerated micro-centrifuge. This first amplification of virus was used to infect HEK-293T cells plated overnight at 5x106 cells/T-150 flask. The growth medium of the cells in the T-150 flasks was changed to 18 ml 5% FBS 1 hour prior to infection. The next day, 18 ml of medium + 10% FBS was added and the cells were cultured for an additional 2 days when most of the cells had rounded up and were observed to be GFP positive by fluorescence microscopy. The cells were lysed by 5 freeze/thaw cycles in 5 ml of culture supernatant and centrifuged for 10 minutes at 2000xg in a refrigerated micro-centrifuge. The supernatant was stored at -70˚C. Quiescent HSC were infected with pAdeno (TGF-β) GFP A T or pAdeno GFP as control at an MOI of 100. After 24-72 hours, GFP expression was determined by FACS cytometry.
The first cell division of single LTR-HSC is accelerated in the presence TGFβ-MAB
LTR-HSC incubated with the TGFβ1,2,3 neutralizing monoclonal antibody 1D11.1613,14 (designated as “TGFβ- MAB”) plus combinations of IL-3, IL-6, SCF in “medium” (see M&M) entered their first cell division more rapidly as detected by direct microscopic observation (Figure 1). At day 1, essentially no cell division was observed, however by day 2, ~30% of all single cells had divided in the combination of IL-3, IL- 6, SCF, while inclusion of TGFβ-MAB increased the proportion of cells undergoing their first cell division rose to ~70% (Figure 1). Cloning efficiency of the LTR_HSC was very high, eventually reaching 100% in the cytosine combinations tested. Trikingly, essentially all single LTR-HSC cultured with SCF+IL-6 eventually underwent their first cell division but it could take up to 14 days (Figure 1). Inclusion of TGFβ-MAB to SCF +IL-6 reduced first division time by half (~7 days).
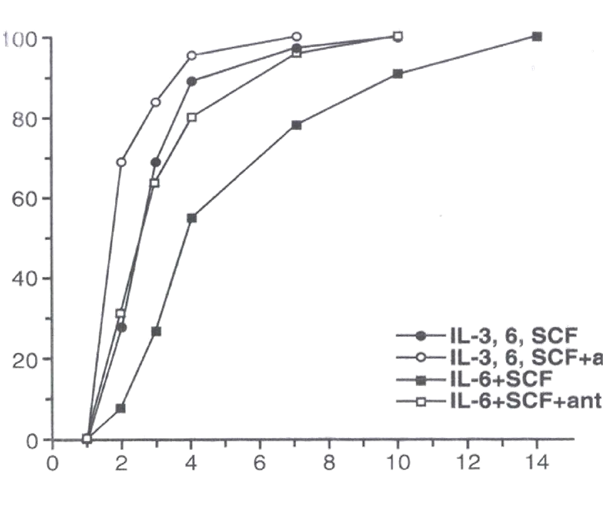
Figure 1 The first cell division of single LTR-HSC mediated by growth factors is accelerated in the presence TGFβ-MAB. Data points indicate % single cells that have undergone their 1st cell division at each day (1-14d) post T=0.
Single LTR-HSC were sorted directly into 96 well round-bottom plates containing “medium” (see M&M), SCF (50ng/ml), IL-6 (20 ng/ml), IL-3 (50 ng/ml) +/- 20 ug/ml TGFβ1,2,3 neutralizing MAB (1D11.16) as indicated. The results are expressed as a mean of the number of wells in which 2 cells were first observed.
LTR-HSC cultured in serum containing medium without added growth factors survive for extended periods in the presence of TGFβ-MAB
LTR-HSC in medium alone (serum containing or serum-free) died within 3 days in culture. However, LTR-HSC plated at 45+/2 per well in the presence of decreasing concentrations of 1D11.16, LTR-HSC survived (were highly light refractive and trypan blue negative) for extended time periods depending on the concentration of the TGFβ-MAB (Figure 2A). No cell division was observed in the presence of TGFβ-M AB in the absence of added growth factors. When single LTR-HSC/well were cultured with an isotype MAB (IgG1K) only the 1D11.16 MAB increased LTR-HSC survival (Figure 2B).
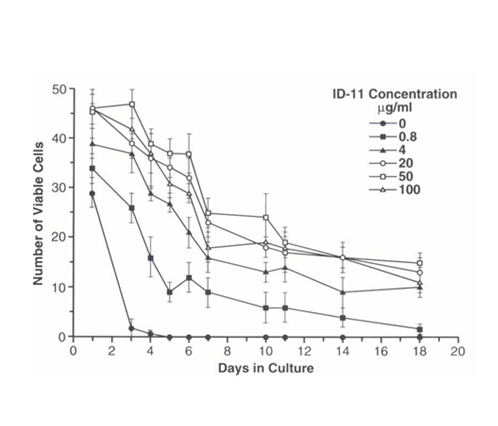
Figure 2A LTR-HSC cultured in serum containing medium alone survive for extended periods in the presence of TGFβ-MAB.
Survival of LTR-HSC cultured in “medium alone” is correlated and dependent on the concentration of 1D11.16 MAB. LTR-HSC (45 cells per well) were cultured with varying concentrations of ID11.16 antibody. At the indicated days, viable cells were determined trypan blue exclusion and degree of light refraction by direct microscopy (200X magnification). Values are given as mean cell number of 4 replicate wells +/- SE.
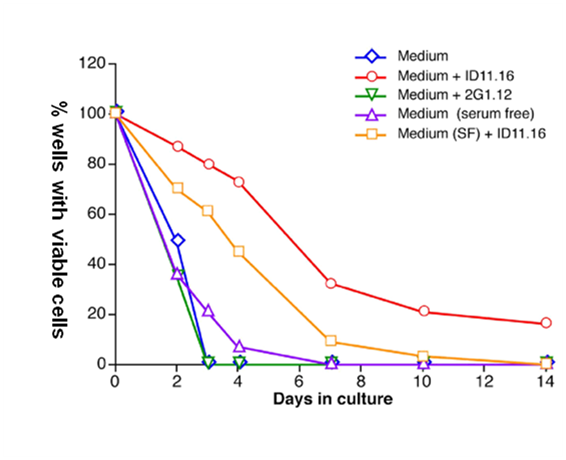
Figure 2B Factors in serum potentiate the TGFβ1-MAB mediation of survival of single LTR-HSC.
Anti-TGF-β antibody 1D11.16 increases survival of single HSC in serum-free media without added growth factors. Single LTR-HSC were directly sorted into round bottom 96 well plates containing serum or serum-free medium +/- 1D11.16 (20 ug/ml), or 2G1.12 (20 ug/ml, a control MAB that binds to TGF-β2 but does not inactivate it). At the indicated day viable cells were determined by the addition of SCF, IL-3, and IL-6 and 10 days later colony growth was determined (wells containing >10 cells were scored as containing a viable cell at the time point. In general, >90% of single cells formed HPP macroclones at day 10). Each combination was tested using 80 wells (single cell/well).
Factors in serum potentate the TGFβ1-MAB mediation of survival in single LTR-HSC without added growth factors
Increased cell survival was observed in serum free media (QBSF-58) as well as serum-containing media in the presence of TGFβ1-MAB (Figure 2B) indicating that the survival was a direct effect of endogenous TGF-β neutralization and was neither cell-cell nor paracrine mediated.
TGFβ1-PMO ligand or TGFβ type 2 Receptor-PMO results in extended LTR-HSC survival in the absence of growth factors
Table 1 shows an alternative approach: antisense PMO inhibition of TGFβ1 in LTR-HSC. Previously, we have shown that un-charged, 18-21-mer PMOs targeted to the transcription start site of the target gene are taken up by highly purified LTR-HSC at 40ug/ml (data not shown). Single LTR-HSC were sorted directly into 96 well round-bottom plates containing TGFβ1-PMO in serum containing “medium”. At day 5, viable cells were determined by their proliferative response to added SCF, IL-6 and IL-3. The proportion of single cultured LTR-HSC that survived in PMOs directed at TGFβ1 mRNA or type II TGF-β receptor mRNA was markedly increased compared to the scramble control-PMO. The TGFβ1-PMO results replicates the TGFβ-MAB result, but unexpected was the type II TGF-β receptor- PMO result which suggests that downstream signaling from TGFβ type ll receptors is implicated in mediating the survival response in the absence of growth factors.
Treatment |
Proportion Single LTR-HSC1 Viable2 at day 5 |
None-medium alone |
0% (0/81) |
Scrambled control-PMO |
2% (2/78) |
TGFβ1-PMO ligand |
39% (27/69) |
TGFβ Receptor Type ll- PMO receptor |
29% (23/79) |
Table 1 TGFβ1-PMO and TGFβ- (Type II receptor)-PMO directly promote survival of single LTR-HSC in the absence of growth factors
Endogenous TGFβ1 expression in Lin- Sca-1+ cells is inhibited by TGFβ-MAB or TGFβ1-PMO
We next wanted to verify that endogenous TGFβ expression is actually inhibited by TGFβ-MAB or TGFβ1-PMO incubation. In order to obtain sufficient number of cells for these studies, we used Lin- Sca-1+ cells for these studies, which are enriched for both LTR-HSC (~10% of cells) and STR- HSC (~90% of cells)20-23 and Bartelmez (unpublished). When single lin- Sca- 1+ cells from C57B6 mice were tested for survival in the absence of growth factors they also showed an increased survival when incubated with ID11.16 (60% vs. 20% survival at day 5, data not shown). Our previous studies also showed that STR-HSC survive in medium alone, or + 1D11.16, to a greater degree than LTR-HSC (Bartelmez, unpublished results). These experiments used PE-labeled mouse anti-human TGFβ antibodies developed for intracellular detection.
Figure 3A shows inhibition of endogenous TGFβ1 expression in lin- Sca1+ cells from wild type mice before and after TGFβ-MAB (1D11.16, 90.1% +cells reduced to 23.8% + cells) or TGFβ1-PMO incubation (51.2% +cells reduced to 14.6%+ cells). We also quantitated the cell surface expression of type II TGF-β receptors on lin- Sca-1+ cells (>98% positive). Importantly, lin- Sca-1+ cells from TGF-β1 knockout mice (tgf-β1-/-) expressed essentially no type II TGFβ receptors by FACS, indicating that in the absence of TGF-β1 expression, its type II receptor down- regulates (data not shown, Ruscetti).
GFP driven by a TGFβ1 promoter (Ad5-TGF-b-GFP) in lin- Sca-1+ cells is inhibited by TGFβ-MAB
Using another approach to verify the above results, we used an adenoviral vector with a TGFβ1 promoter that drives GFP expression. Transduced lin- Sca1+ cells were incubated with TGFβ-MAB (1D11.16) for sixteen hours. Figure 3B shows that TGFβ expression (GFP fluorescence) was markedly reduced by TGFβ-MAB.
LTR-HSC incubated with TGFβ-MAB for 2 hr or 5 days resulted in similar LTR-HSC donor repopulation
Table 2 shows the result of TGFβ inhibition on the repopulating ability of LTR-HSC during time in culture. Lethally irradiated mice (950 rads) were rescued with 4 x 105 unfractionated (competitor/support) bone marrow cells to allow donor LTR-HSC chimeras to be measured over time. LTR-HSC cultured for 2 hr or five days with TGFβ-MAB in the absence of growth factors exhibited a similar repopulating ability to that of freshly isolated LTR-HSC. Other experiments further verified the profound effect of a 2 hr incubation of LTR-HSC with TGFβ- MAB on donor repopulation (Figure 4).
Conditions |
Cells Transplanted Per Mouse |
Number HPP-CFC |
Donor Cell Repopulation 1.5 Months Post-transplant |
CRU/ 105 1.5 Months Post-transplant |
% Donor Cell Repopulation 10.5 Months Post- transplant |
CRU/105 10.5 Months Post-transplant |
100 LTR-HSC T=0 TGFβ-MAB NO CULTURE |
100 LTR-HSC |
92+/-3 |
48+/-12% |
2803+/-398 |
30+/-29 |
1286+/-1225 |
100 LTR- |
0.8 +/- 0.83 cells
|
0
|
0%
|
0
|
0
|
0
|
100 LTR-HSC |
40+/-6 cells
|
33+/-5
|
25+/-20%
|
1875+/-1646
|
22+/-17
|
1536+/-742
|
Table 2 The Repopulating ability of LTR-HSC cultured for five days with TGFβ-MAB is similar to freshly isolated LTR-HSC
LTR-HSC from CD 45.1 mice were deposited directly from the sorter into 96-well plates with“medium” containing +/- TGFβ-MAB (ID.11.16) or isotype control antibody (IgG1K). At T=0, 10 wells were counted and assayed for high-proliferative potential (“HPP”) formation in agar. At 5d in culture 10 wells containing 1D11.16 and 10 wells containing IgG1K were counted and assayed for HPP formation. At T=0 and T=5d 10 wells each condition was harvested and transplanted with 4x105 CD45.2 competitor/support cells CD 45.2 into 950 rad lethally irradiated. Recipient animals were analyzed for the presence of donor type cells in the blood by FACS analysis. The results from the transplantation assay could be expressed as repopulating units (CRU) per 105 and calculated as described by Harrison et al.26
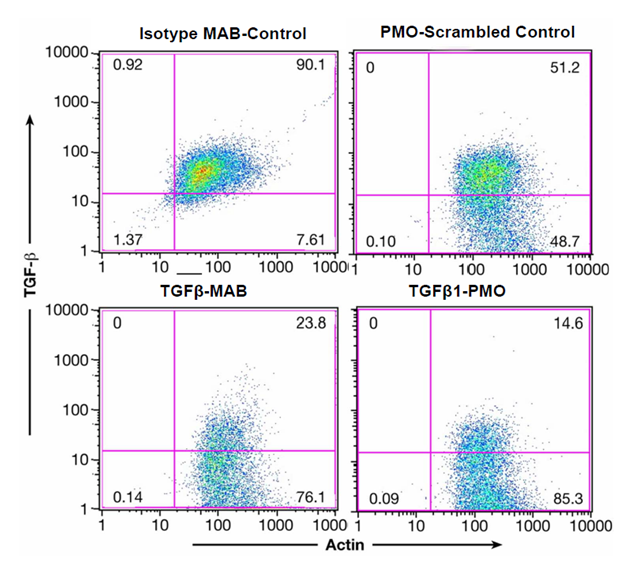
Figure 3A Endogenous TGFβ1 expression in lin-Sca1+ cells is inhibited by TGFβ-MAB or TGFβ1-PMO.
Left panel: lin-Sca1+ cells were isolated as described15 and a 0.5 X 106 cells/ml were cultured overnight in” medium” + 20ug/ml 1D11.6 or 20 ug/ml isotype control. Right panel: lin-Sca-1+ were incubated overnight with 40 ug/ml TGF-β PMO or PMO scrambled control. Intracellular staining for TGFβ1 and actin was performed as described.15 Both TGFβ-MAB and TGFβ1-PMO decreased endogenous TGFβ1 expression in the lin-Sca1+ cells.
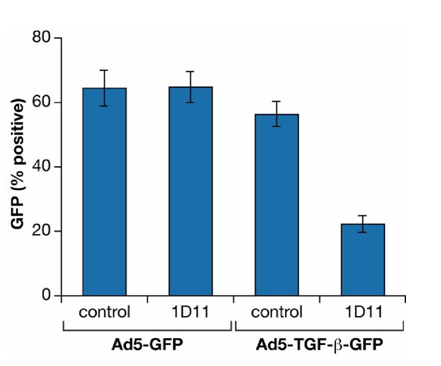
Figure 3B GFP driven by a TGFβ1 promoter construct (Ad5-TGF-b-GFP) in Lin- Sca-1+ cells is inhibited by TGFβ-MAB.
Lin-Sca-1+ HSC were transduced with pAdeno (TGFβ) GFP A T or pAdeno GFP as control at an MOI of 100.
Cultures were incubated for 16 hours at 100,000 cells /200ul in “medium” containing 1D11.16 or IgG1K isotype control. After 24-72 hours, GFP expression was determined by FACS.
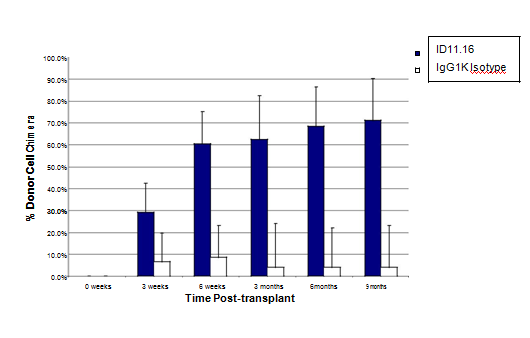
Figure 4 TGFβ-MAB incubation (2 hr) with LTR-HSC induces a rapid and sustained donor engraftment in lethally irradiated congenic mice: LTR-HSC purified from B6SJL mice (CD45.1+) were transplanted iv into lethally irradiated (950 rads) congenic C57Bl6 (CD45.2) mice along with 400,000 unfractionated bone marrow support/ competitor cells (CD45.2). The open bars (+/-S.D. n=14) show the engraftment of 100 LTR-HSC control cells (incubated with IgG1K antibody). The repopulation kinetics of untreated LTR-HSC were similar to that of IgG1K antibody treated LTR-HSC (not shown). The filled bars (+/-S.D. n=19) show the engraftment of 100 LTR-HSC treated cells with ID11.16 for 2 hours just prior to transplant.
LTR-HSC incubated with 1D11.16 MAB or 12H5 MAB repopulate recipient mice with the same kinetics and similar degree of chimerism: comparison to neutralizing MAB to TGFβ2 or TGFβ3
Figure 5 shows that 12H5 MAB which immunoprecipitants only TGF-β1, compared to 1D11.16 which immunoprecipitants TGFβ1 and TGFβ2, however both MABs promoted both rapid and increased long-term donor engraftment of LTR-HSC (Figure 5). Furthermore, Fab’2 fragments of 1D11.16 (generously provided by Bruce Blazar17 also promoted rapid and increased long-term engraftment after a 2-hour exposure to LTR- HSC, indicating that the Fc portion of the antibody was not necessary for the increase engraftment. As mentioned above, the 3C7.14 MAB which immunoprecipitants TGFβ2 did not promote LTR-HSC to increase their donor engraftment, however 20724 MAB which neutralizes TGFβ3 did induce LTR-HSC to engraft but resulted in only short term repopulation.
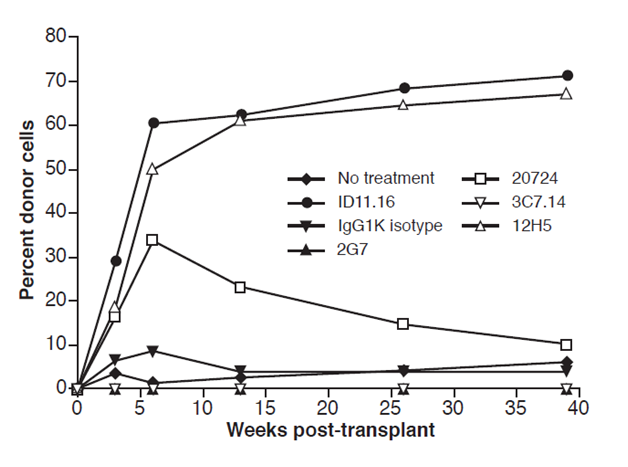
Figure 5 LTR-HSC incubated with 1D11.16 MAB or 12H5 MAB repopulate recipient mice with the same kinetics and same degree of chimerism. 1D11.16 MAB (TGFβ 1,2,3 neutralizing MAB and supported LTR-HSC survival in vitro single cell cultures) or 12H5 MAB (TGFβ1 neutralizing MAB and supported LTR-HSC survival in vitro single cell culture). Additionally, LTR-HSC were incubated with IgG1K (isotype MAB: did not support LTR-HSC survival in vitro single cell cultures), 2G7 (TGF-β1,2,3 MAB: did not support survival of LTR-HSC in vitro single cell cultures), 3C7.14 (TGFβ2 MAB: did not support LTR-HSC in vitro survival), no treatment: did not support LTR-HSC in vitro single cell survival.
LTR-HSC incubated with 1D11.16 or Fab’2 or TGFβ1-PMO generate a large proportion of neutrophils during the early period after transplantation
Figure 5 shows another unexpected result, only observed when TGFβ1,2 MAB (1D11.16) or the Fab’2 fragment of 1D11.16, or TGFβ1-PMO was present in the incubation prior to transplant: a dramatic increase in the proportion and number of donor neutrophils during the first month post iv transplantation of LTR-HSC.
LTR-HSC incubated with TGFβ-MAB rescue lethally irradiated mice from hematopoietic death
Next, direct transplants (without competitor/support marrow) showed unexpectedly that low numbers of LTR-HSC treated with TGFβ-MAB (1D11,16) could rescue a lethally irradiated mouse from hematopoietic death (Figure 6). Strikingly, few as 60 LTR-HSC treated with ID11.16 and transplanted within 2 hours, 60-70% of mice recovered and survived for at least 6 months. In contrast, 60 LTR-HSC incubated with isotype MAB (IgG1K) resulted in which had no surviving mice. Essentially all mice that received between 250-1000 anti-TGF-β treated LTR-HSC survived compared to the controls in which survivors were first observed only at 1000 LTR-HSC per mouse and then only10-20% survived. Both the TGFβ-MAB and TGFβ1- PMO treated LTR-HSC could rescue lethally irradiated mice from irradiation induced hematopoietic death Table 3 and provide long-term engraftment without support marrow in striking contrast to the isotype antibodies and scrambled PMO.
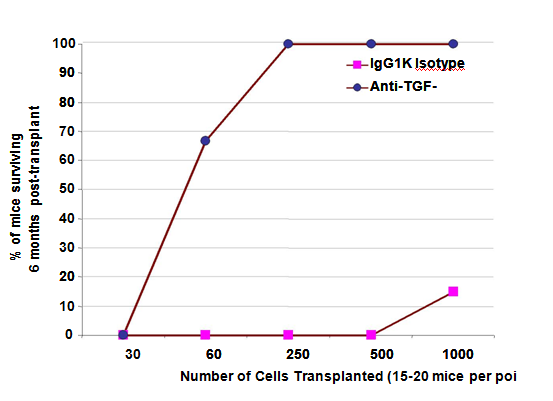
Figure 6 LTR-HSC incubated with TGFβ-MAB rescue lethally irradiated mice from hematopoietic death.
Hematopoietic death occurs in essentially 100% of mice after 950 rad lethal irradiation. Shown is a SURVIVAL AT 6 MONTHS dose response curve after transplant of CD45.1-LTR-HSC. Lethally irradiated mice received 30, 60, 250, 500 or 1000 LTR-HSC per mouse +/- TGFβ- MAB incubation just prior to transplant. (n=20 mice at 30250 cell dose and n=15 mice at 500-1000 cells). As few as 60 LTR-HSC incubated with 20 ug/ml 1D11.16 MAB rescued 60-70% of mice from hematopoietic death without competitor/support marrow. 100% of lethally irradiated mice survived if they received 250, 500 or 1000 LTR-HSC treated with 20ug/ml1D11.16 MAB just prior to transplant. Untreated LTR- HSC were unable to rescue lethally irradiated mice up 500 LTR-HSC per mouse, however at 1000 untreated LTR-HSC/ mouse 10% survival was observed.
Enhanced transplantation efficiency requires an increased ability for HSC to rapidly short-term repopulate without depleting the pool of LTR-HSC. At non-limiting doses and in non-competitive assays, treatment of HSC with TGFβ- MAB resulted in survival, accelerated engraftment and generated durable long-term donor engraftment producing chimeras of up to >90% donor cells while transplanting only sixty TGFβ- MAB treated LTR-HSC. Overcoming hematopoietic death in the absence of support marrow requires that donor derived platelets and neutrophils be rapidly generated post- transplant.16,25 LTR-HSC treated with TGFβ-MAB or TGFβ1-PMO skewed early reconstitution toward favoring neutrophils over lymphoid recovery in both support and direct transplants (Table 4). Platelet regeneration was not directly measured, but the rescue of mice strongly suggests that platelets recovery was rapid as well. Delayed production of neutrophils and platelets is a major problem in adult cord blood transplantation leading to high morbidity.2-5 Competitive/support repopulating assays provide a functional assessment of experimental donor HSC compared to constant numbers of competitor cells.26 Transplant of 100 TGFβ- MAB treated LTR-HSC generated a long-term engraftment of 70-90% donor cells depending on the number of LTR-HSC transplanted. The mechanism (s) meditating the profound effects on LTR-HSC survival in the absence of growth factors and the rapid and enhanced engraftment in irradiated recipients remains unclear but are likely to be multifactorial. Injecting HSC directing into the marrow cavity has been shown to improve engraftment compared with intravenous administration.27,28 Removal of CD26 peptidase activity from the surface of HSC leads to increased transplantation efficiency through at least in part increased HSC homing.1 HSC treated with SDF-129 or HSC engineered to overexpress CXCR430 have also been shown to increase engraftment. Interestingly, exposure of cord blood CD34+ cells to low levels of TGFβ1 upregulated CXCR4 surface expression and increased migration and adhesion responses in the presence of SDF-1, which downregulates CXCR4.31 Our current studies also show that TGFβ-MAB treatment increases migration of murine lin- c-kit+/Sca-1+ and human CD34+ cells to SDF-1 in Boyden chambers.15 Physiologic inhibitors of active TGFβ such as decorin, biglycan and fibromodulin released by marrow stromal cells have been described.32 Physiologic proteases that can activate latent TGF β have been described such as tissue transglutaminase expressed by HSC and stromal cells33 (and Bartelmez unpublished results). Previously we demonstrated that TGFβ1,2 inhibition was a reversible regulator of LTR-HSC quiescence7 and thus physiologic inhibitors of active TGFβ and activators of latent TGF-β could play a central role in this regulatory pathway.
The role of cell cycle position on HSC bone marrow engraftment has been studied extensively and both murine and human HSC bone marrow engraftment with greater efficiency at the G0/G1 phase of the cell cycle, in contrast to the low engraftment of HSC observed in the G2/S/M phase.34-37 Studies by Dao et al.38 showed that bone marrow CD34+ cells exposed to SCF, IL-3, IL-6 plus neutralizing TGFβ antibodies for 48 hour had more CD34+ cells in G1 than CD34+ cells cultured in SCF, IL-3, IL-6 alone. Furthermore, they clearly demonstrated through transplant studies that CD34+ cells cultured with growth factors and TGFβ antibodies for 48 hours had the same repopulating ability of CD34+ cells cultured in growth factors alone. Generally, our transplant studies did not include a culture period with the exception of 16hr incubation with TGFβ1-PMO or LTR-HSC survival studies both of which did not include growth factors. Human bone marrow CD34+ cells have been shown to remain mitotically quiescent for up to 72 hours after transplant into NOD/SCID mice.37 Therefore, our further studies will include the effect of TGFβ1-PMO treated LTR-HSC on their state of quiescence after homing and engraftment into the bone marrow. TGFβ1- PMO treated LTR-HSC may undergo more self-replication proliferative cycles once engrafted. Studies show that inhibiting TGFβ mediated Smad signaling by overexpressing the inhibitory Smad 713 increased HSC self-renewal. Thus, inhibiting Smad signaling may have a cascade effect on HSC self-renewal and homing. Finally, the differential effects of TGFβ-MAB vs. TGFβ1-PMO must be considered.
In both approaches, TGFβ endogenous expression in HSC was downregulated, however the downregulation by TGFβ-MAB is dependent on inhibiting LTR-HSC surface expression of TGFβ ligand thus preventing binding of TGFβ ligand to its receptor. TGFβ1-PMO acts by an irreversible binding to TGFβ1 mRNA which inhibits TGFβ1 mRNA translation to protein. Interestingly, TGFβ1-PMO treatment of LTR-HSC could induce 1- 2 cell divisions within 16 hr, in contrast to TGFβ-MAB neutralizing antibodies where cell division was not observed, even after many days in culture (Table 3). Other antisense studies have shown reduced intracellular TGF-β/Receptor complexes.39 Endogenous TGFβ expression in lin-Sca-1+ cells from TGFβ1 knockout mice (tgfβ1-/-) indicated no detectable TGFβ1 or TGFβ2, and intracellular staining of wild-type lin-- Sca-1+ cells showed that endogenous TGFβ expression is predominantly TGFβ1 (Ruscetti, data not shown). Furthermore, previous studies have shown that lin-- Sca-1+ cells incubated with TGFβ-MAB effectively downregulated the endogenous expression of TGFβ1 which autoregulates its transcription.24 However, intracellular signaling by TGFβ is not completely understood. In summary, these data show marked improvements in HSC transplantation efficiency in experimental animal models and suggests that this approach can be clinic useful in settings of limited donor HSC. Other recent studies have demonstrated that inhibition of endogenous TGFβ1 in human CD34+ cells can substantially reverse the dysfunctions of diabetic lin-CD34+CD45+ stem cells41 and dysfunctional CD34+ cells isolated from Fanconi anemia patients.42
Treatment |
Number of Cells Transplanted |
Proportion of Mice Surviving Surviving Lethal Irradiation |
% CD 45.1 Donor Chimera in Surviving Mice |
||||
|---|---|---|---|---|---|---|---|
Day 0 |
Day 1 |
3 weeks |
6 weeks |
3 months |
6 months |
||
Isotype MAB |
60 ± 0.5 |
ND |
None (0/12) |
NA |
NA |
NA |
NA |
TGFβ-MAB (1D11.16) |
60 ± 0.5 |
ND |
66% (6/9) |
74 ± 10 |
87 ± 6 |
92 ± 10 |
89 ± 7 |
TGFβ1-PMO |
ND |
105 ± 0.5 |
75% (9/12) |
51 ± 18 |
77 ± 13 |
87 ± 14 |
90 ± 12 |
Control-PMO |
ND |
60 ± 0.5 |
None (0/12) |
NA |
NA |
NA |
NA |
Table 3 TGFβ-MAB or TGFβ1-PMO promote rapid engraftment of low numbers of LTR-HSC (without competitor/support marrow cells) and generate high donor chimeras
Recipient mice (CD45.2) were lethally irradiated (950 rads) and were transplanted iv with LTR-HSC (CD45.1) that had been incubated with either TGFβ-MAB (1D11.16) or isotype MAB (IgG1K) 2 hour prior to transplant or incubated for 16 hr with 40ug/ml TGFβ1PMO or a control scrambled-PMO. No competitor/support cells were used in these experiments. ND=Not done. NA= no surviving mice.
Treatment |
1 Month Post- Transplant |
Number of Transplanted Cells T=0 |
% CD45.1 Donor Cells |
% CD45.1 Donor Neutrophils |
% CD45.1 Donor B Lymphocytes |
% CD45.1 Donor T Lymphocytes |
None |
competitor/support |
100 ± 12 |
8 ± 4 |
2.8 ± 2 |
80± 25 |
18 |
Isotype MAB (2hr) |
competitor/support |
100 ± 12 |
6.8 ± 4 |
2 ± 2 |
80± 19 |
16 |
TGFβ MAB (2hr) |
competitor/support |
100 ± 12 |
42 ± 8 |
92 ± 5 |
7± 3 |
1 ± 2.1 |
TGFβ MAB (2hr) |
direct transplant |
60 ± 5 |
74 ± 10 |
91 ± 7 |
6± 5 |
1 |
TGFβ Fab’2 MAB (2hr) |
direct transplant |
60 ± 5 |
59 ± 14 |
83 ± 11 |
9± 7 |
1 |
TGFβ1 PMO (16hr) |
direct transplant |
120 ± 10 |
51 ± 18 |
88 ± 6 |
9± 4 |
1 |
Table 4 LTR- HSC incubated with 1D11.16 or Fab’2 1D11.16 or PMO generate a large proportion of donor neutrophils during the early period after transplantation
Recipient mice (CD45.2) were lethally irradiated and were transplanted iv with 100 LTR-HSC (CD45.1) that either had no treatment (none) or a 2 hr incubation at 37˚C with isotype antibody IgG1K, or 1D11.16, or Fab’2 fragment of 1D11.16 or TGFβ1-PMO. “competitor/support” indicates the LTR- HSC (CD45.1) were transplanted along with 400,000 unfractionated bone marrow (CD45.2). Direct transplant indicates only LTR-HSC (100 per mouse) were transplanted (no competitor/support cells). Donor cell chimeras were identified using flow cytometry as cells expressing CD45.1. Neutrophils were defined as 7/4+ cells, B-lymphocytes as B220+ cells, T-lymphocytes as CD4 and CD8 cells.
This work was primarily supported by grant RO1- DK48708, from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (SB), and AVI-Biopharma Inc (aka Sarepta Therapeutics) Corvallis, OR. This research was also supported in part by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, NIH (FWR). We are especially grateful to Elizabeth Bartelmez for her patience editing this manuscript and more importantly, her strong support over the several years that this work was developed.
The author declares no conflict of interest.

©2016 Bartelmez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.