Journal of
eISSN: 2475-5540


Mini Review Volume 1 Issue 2
Centre for Nanotechnology & Regenerative Medicine, Division of Surgery & Interventional science, University College London, United Kingdom
Correspondence: Alexander M Seifalian, Professor of Nanotechnology and Regenerative Medicine, University College London & Co-Director of NanoRegMed Ltd, London, United Kingdom, Tel 44 7985380797
Received: September 23, 2015 | Published: January 11, 2016
Citation: Seifalian AM, Radenkovic D. Next generation treatment of diseases and injury is stem cells therapy. J Stem Cell Res Ther. 2016;1(2):44-47. DOI: 10.15406/jsrt.2016.01.00008
Stem cells can offer definite cure for many diseases and regeneration of failing human organs, but so far researchers have been unable to exploit their full potential. Stem cell therapies are not yet established treatments and are currently in clinical trials. Adult bone marrow or adipose tissue-derived mesenchymal stem cells are most commonly used, even though both adult and embryonic stem cells are available. The main application of stem cells will be either in developments of organs or stem cells therapy.
Organ development has been based on a 3D scaffold made of biological or synthetic materials, seeded with stem cells in a bioreactor. Although biological materials were initially used, synthetic nano materials are more likely to enable “off-the-shelf” tissue-engineered organs. The world’s first synthetic trachea was successfully implanted into a patient as a compassionate case.
Most trials using stem cell therapy were conducted for cardiac or neuronal regeneration. Initially, treatment involved direct injection of stem cells into patients, site injection or systemic administration, and had variable success. Novel approach is to administer stem cells with specialized scaffolds, growth factors and chemokines to stimulate stem cell proliferation and guide them to the target organ. Use of scaffolds as well as bioactive molecule and materials for capsulation of stem cells, enhances viability and provides better control of stem cell fate in the human body post-delivery.
Stem cells representanincredibly promising field of medicine. Clinical trials are now required to make techniques initially used as compassionate cases, readily available as viable clinical treatments.
Keywords: stem cells, tissue engineering, nanocomposite, organ grafts, regenerative medicine
GLP, good manufacturing laboratory protocol; POSS-PCU, polyhedral oligomeric silsesquioxanepoly(carbonate-urea)urethane
Stem cells have huge potential for treatment and regeneration of human organs. There is a large number of researchers working towards a better understanding of their basic behavior and differentiation to mature cells. At the same time, numerous scientists and clinicians are pushing for treatment of diseases using stem cell therapy. These include diseases or injury to the skin, heart muscle, trachea, cartilage, bone and surface of the eye. In addition, there are large numbers of clinics around the world offering stem cell therapy for numerous diseases and injuries.
Currently all these therapies are at an early stage, either part of a clinical trial, or are not yet established treatment. Therefore, it is not clear who will perform the therapy, commercial companies, hospitals, or dedicated institutes.
A large amount of work has been committed to different sources of stem cells. Stem cells can be broadly divided into adult stem cells and embryonic stem cells. Adult stem cells isolated from an aspirate of patient’s own bone marrow or adipose tissue are already in widespread use. These are known as “autologous” as they have been extracted from patient’s own tissues. Most commonly used adult stem cells are bone marrow stromal cells, also referred to as bone marrow mesenchymal stem cells, as they are readily available and have the potential to differentiate into multiple cell types. In addition, adipose-derived mesenchymal stem cells are also getting popularity in regenerative medicine, as well as in cosmetic used by plastic surgeons.1 They can be isolated in larger quantities than bone marrow mesenchymal stem cells and by significantly less invasive procedures.2
Although one may reasonably assume the use of autologous stem cells is absolutely safe, as it does not carry risk of immune rejection, this is not always the case. Autologous adipose-derived mesenchymal stem cells are often isolated from adult adipose tissue with the aid of collagenase digestion and subsequent centrifugation. Administration of isolated stem cells to the patient at this multi-potent stage is considered safe. However, if the cells are grown in cell culture in clinical laboratories in order to differentiate and acquire characteristics of mature cell types, and afterwards reintroduced into the patient after several passages, the procedure carries certain risks.3 These include contamination with bacteria and viruses, which can cause disease when in the body. Therefore, the cells should be manufactured with high standard of quality control at Good manufacturing Laboratory (GLP) standards, and sufficient funds and regulation must achieve this.
Embryonic stem cells are isolated from the embryo blast (inner cell mass) of the blastocyst usually collected from unwanted embryos created by In Vitro Fertilization. They are easier to grow in cell culture and could find more diverse clinical applications, because they are pluripotent and can differentiate into cells of all three germ layers: endoderm, mesoderm and ectoderm. On the other hand, they could cause immune rejections and their proliferation and differentiation are more difficult to control.4 Thus they could form tumors when introduced into patients unless made fully responsive to their environment.
Many countries have strategic plans, making stem cell science a national priority and supporting it with large funding. These include USA, Japan, Canada and many other countries. There is greater awareness of stem cell research among general population, and increasingly, parents from all over the world are storing umbilical cord blood rich in stem cells for future use. Currently, they can be used to treat patients with hematological malignancies, immunodeficiency and bone marrow failure. As the knowledge of stem cell application is expanding, more conditions that can be successfully treated with cord blood stem cells will be added to the list.
The main application of stem cells in treatment of disease and injuries will be either in the development of organs to replace damaged organs, or stem cell therapy to regenerate damaged tissue in the organ.
Development of organs using stem cells
Organ development have been based on a 3D scaffold, made either from non-biodegradable materials or bio absorbable materials which are coated with stem cells in a bioreactor.5 There are numerous benefits of using autologous stem cells for coating, as there is no need for severe immune suppression. Life-long immune suppression, as in traditional allo-transplantation, increases the risk of infections and post-operative complications.6 The scaffold materials used have been either synthetic or biological. The scaffold is as important as stem cells, and interaction of scaffold and stem cells is crucial for successful outcome.
Biological materials include extracellular matrix proteins taken from mammalian tissues previously decellurized and chemically treated to remove characteristic antigenic epitopes, which could provoke an immune response. In more recent years, a lot of attention was drawn to the preservation of amniotic membrane for potential use as a biological scaffold for tissue engineering, after it was successfully used as a wound dressing and shown to promote healing in patients with necrotizing fasciitis.7 Amniotic membrane has antibacterial properties and with novel inexpensive techniques to remove existing epithelial cells and fibroblasts, but still keep extracellular matrix proteins that promote cell adhesion and proliferation, it could find large-scale application, particularly for skin and small intestine grafts.8
In the first in human transplantation of a tissue-engineered airway, was 30-year old Claudia Castillo with life-threatening stenosis of the left main bronchus in,9 trachea from a cadaver donor was decellurized and then coated with patient’s own epithelial cells and chondrocytes derived from bone marrow mesenchymal stem cells.10 The grafts were very short and mechanical properties were less important. Similar procedures of implanting decellurized allogeneic trachea from a donor, seeded with autologous stem cells and growth factors, was repeated in the UK in a 10-year old boy with congenital tracheal stenosis and pulmonary artery sling. Even though the graft initially lacked adequate mechanical stability, vascularization and growth of the implant occurred 18 months post-transplantation, and the boy showed full functional recovery and normal lung function.6
Despite the extraordinary success of the first in-patient transplantations using this technique, some scientists still believe that it will not be able to allow widespread life-saving treatment of patients, mainly because of limited number of appropriate organ donors and risks of prion-related diseases. Use of synthetic materials for 3D scaffold, particularly nanomaterials, may offer a solution and enable “off-the-shelf” tissue-engineered organs. There are large number of nanomaterials with large surface area and nanotopography, which are ideal for adhesion, proliferation and differentiation of stem cells to mature cells. Their porous structure is of fundamental importance, as it must allow cell seeding but still be small enough to provide sufficiently large surface area. In addition, specific nanomaterials could be designed to guide stem cell fate with growth stimulating factors and other chemical cues.
Example of synthetic material is the bioabsorbable Nanocomposite polyhedral oligomeric silsesquioxanepoly (carbonate-urea) urethane (POSS-PCU), which underwent full toxicology studies under GLP and is already safely used in the clinic. Since it was patented by Seifalian & co-workers in,7 it has been successfully used for the synthesis of vascular grafts, stent coating, calcification-resistant heart valve prostheses, lacrimal ducts and tracheal grafts. Patient with first engineered lacrimal duct using POSS-PCU scaffold is under follow up.11 Moreover, it was shown that human adipose mesenchymal stem cells derived from pediatric patients differentiated into chondroblasts in POSS-PCU scaffolds, with consequent successful vascularization of the bionanocomposite. Cartilage grown in this way on POSS-PCU scaffold could be used for ear cartilage repair for children with congenital auricular malformations and other cranio-facial defects, especially in cosmetic surgery.12 Bone marrow mesenchymal cells were also able to undergo chondrogenesis on POSS-PCU auricular-shaped scaffold13 and reassured the scientific community that tissue-engineered auricle using stem cells, will undoubtedly revolutionize surgical reconstruction of external ear cartilage.
In the world’s first synthetic in-human trachea with two bronchi transplantation, POSS-PCU based Y-shaped tracheal graft was synthesized in suitable size based on patient’s computerized tomography scans. It was used as a synthetic 3D scaffold, and it was functionalized in a bioreactor with stem cells extracted from the bone marrow as shown in Figure 1. This first successful tissue-engineered trachea, implanted in the patient as a compassionate case, showed that with the use of synthetic biocompatible nanomaterials, stem cells organ development in a laboratory is now a real possibility for terminally ill patients with failing organs.14
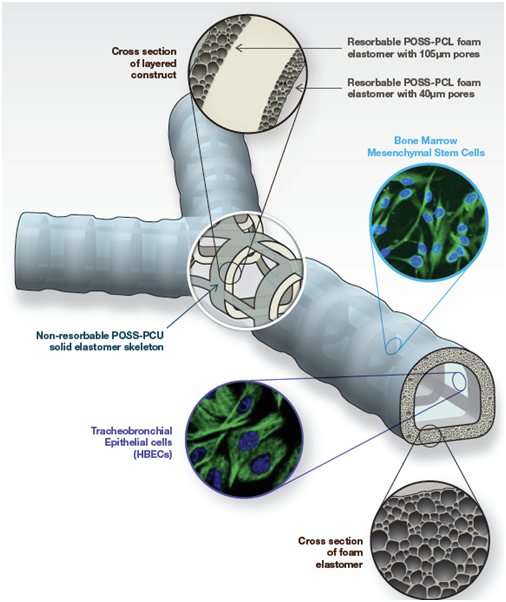
Figure 1A Design of the synthetic tracheal and bronchial scaffold. POSS-PCL, polyhedral oligomeric silsesquioxanepoly (ε-caprolactone) urea urethane; POSS-PCU, polyhedral oligomericsilsesquioxane poly(carbonate-urea) urethane .25

Figure 1B Actual scaffold made from POSS-PCU with autologous bone marrow mesenchymal stem cells implant into patient.
Recent advances have been made in the field of POSS-PCU coronary artery grafts, where incorporation with S-nitrosothiols and consequent release of nitric oxide lead to superior graft patency, improved endothelization and resistance to thrombi formation.15
Skin regeneration using stem cell based tissue engineering is not lagging behind. Skin autografts are the current gold standard, but have major drawbacks due to limited availability in case of severe burns and scar contracture, sensory loss of grafted skin and unsatisfactory aesthetic outcome.16 Deculleruzised human or porcine dermis is already commercially available for use in traumatic injuries, burns and skin damage caused by diabetes, but unless vascularized the tissue would die and serve more as covering than as a regenerator. Combination of autologous stem cells with available dermal substitutes could ensure complete skin regeneration. Bilayered dermal substitute Integra (Integra has deep dermal layer made of bovine collagen and glycosaminoglycans, and the superficial layer synthesized of silicone) seeded with autologous bone marrow mesenchymal stem cells already showed superior vascularization and tissue repair in full-thickness skin-wounds in animal models.17
Stem cell therapy
The other area of application of stem cells in treatment of diseases has been stem cell therapy. Previously stem cells were directly injected into patients, such as in clinical trial of intravenous injection of autologous bone marrow mesenchymal stem cells after myocardial infarction, in the treatment of ischemic myocardium.18 Although stem cells proved safe to use, the results did not reveal drastic cardiac muscle regeneration. Researchers realized that this approach may not work, as percentage cell survival of injected stem cells is low. Novel approach is to use scaffolds as well as bioactive molecule as vehicle for stem cells delivery systems. Currently, large number of researchers is working on incorporation of stem cells with scaffolds and proteins as a method of delivery of stem cells, to ensure mechanical support and increase cell survival, as well as growth factors to guide stem cell differentiation. Change in strategy has already given better outcomes, and latest research on cardiac regeneration using releasing and structural scaffolds and adult stem cells is giving promising results.19,20
Stem cells are currently in clinical trials for neuro regeneration and if successful, they could improve lives of patients with conditions, which lack any treatment plan at the moment, such as acute spinal injury. Partial functional regeneration of the transected spinal cord was achieved after transplantation of autologous olfactory ensheathing stem cells.21 However, in other clinical studies injection of neural stem cells isolated from areas of the brain with life-long neurogenesis, subgranular layer of dentate gyrus of the hippocampus and subependymal zone of the lateral ventricles, to treat spinal cord injury, were not as successful. It was again hypothesised that injection of stem cells directly into patients led to poor integration into host tissue and unresponsive differentiation, due to unfavorable environment and glial scar formation. Once stem cells were seeded on a biocompatible scaffold, much better results were seen. Scaffold material should be of similar mechanical properties to the spinal cord and act as delivery vehicle for growth stimulating factors and reduce scar formation. Natural scaffolds of fibrin and collagen or synthetic poly(α-hydroxy acid) have all been already used. Combinatorial approach of using both tissue engineering and stem cell therapy may be able to finally provide effective regeneration of nerve tissue.22
Embryonic stem cells from both mice and humans were able to form all ten layers of the retina in experimental studies after induction with appropriate signaling molecules.23 Thanks to these promising findings in experimental studies, embryonic stem cells are currently in a clinical trial to prevent blindness caused by age-related macular degeneration, Age-related macular degeneration is the most common cause of blindness in developed countries with no treatment options available. It is caused by destruction of retinal pigment epithelium and consequent functional loss of photoreceptors and blindness. The goal is to use embryonic stem cells to regenerate retinal pigment epithelium before the damage of photoreceptors occurs.24 If successful, stem cells could also be used to treat inherited eye conditions including retinitis pigmentosa, Usher syndrome and Leber Congenital Amaurosis.
It is now well known that environment of stem cells promotes tissue repair and cell regeneration due to paracrine effects and release of growth stimulating factors and nanometer-sized lipid vesicles, exosomes. These could be used therapeutically on their own.
Stem cell therapy is undoubtedly the next generation of treatment for many diseases and the scope of their medical applications is immense. Tissue engineering is no longer a fairy-tale. However, urgent research on suitable scaffold design, and trials in larger number of patients are required to make techniques initially used as compassionate cases, readily available clinical treatments.
None.
The author declares no conflict of interest.

©2016 Seifalian, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.