Journal of
eISSN: 2475-5540


Mini Review Volume 1 Issue 4
Chief Scientist - Managing Director at CuroNZ, New Zealand
Correspondence: Frank Sieg, Chief Scientist - Managing Director at CuroNZ, PO Box: 8123, Symonds Street, Auckland, New Zealand
Received: August 17, 2016 | Published: September 6, 2016
Citation: Sieg F. Mini-review of neural regeneration peptides in brain development. J Stem Cell Res Ther. 2016;1(4):147-148. DOI: 10.15406/jsrt.2016.01.00025
During mammalian and especially human ontogenetic brain development, cues of chemoattractive and chemorepulsive character are interacting in a spatial-temporal manner to direct neuroblasts to the dedicated locations in the neocortex. Finally, neuroblasts differentiate to fulfil their respective function in the neural circuit. Most if not all of these important molecules, that direct neuronal migration, display activity profiles in the nanomolar range. In contrast, the novel gene family of Neural Regeneration Peptides (NRPs) has a neuronal survival and chemo attraction profile in the subpicomolar range which makes the NRPs interesting candidates to represent the main constituents for neuronal chemo attraction during ontogenesis as well as injury or disease states of the brain. This review is focusing on early gene expression of the mouse nrp gene within the cortex as well as on the potential for the drug candidate NRP2945 to provide breakthrough treatment options for chronic disease states of the brain in the future.
Keywords: human ontogenetic brain development, spatial-temporal manner, neural regeneration peptide, chemokine receptor subunit
CAPS-2, calcium dependent activator protein for secretion 2; BDNF, brain derived neurotrophic factor; CXCR4, chemokine receptor with cxc motif 4; GABA, gamma amino-butyric acid; GABA A receptor, gamma amino-butyric acid receptor a; GAERS, genetic absence epilepsy rats from strasbourg; LGS, lennox-gastaut syndrome; MAP-2, microtubule associated protein-2; MOG-EAE, myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalitis; NRP, neural regeneration peptides; NRP2945, neural regeneration peptide 2945; NT-3, neurotrophin-3; PTZ, pentylenetretrazol; RefSeq, reference sequence; SVZa, anterior subventricular zone.
The Neural Regeneration Peptide (NRP) molecule entity belongs to a family of gene encoded proteins which are inherent to all mammals. These NRPs are ultra-potent when used in in vitro neuronal survival, chemoattraction as well as neuronal differentiation assays.1 NRPs are binding as agonists to a novel plasma membrane receptor complex at subpicomolar concentrations. This NRP receptor complex is currently under investigation and consists of CXCR4 and another undisclosed chemokine receptor subunit. CXCR4 activation by NRP leads to increase of GABA A receptor subunit (alpha and beta) protein expression and upregulation of GABA.2,3 In this mini-review the company CuroNZ has investigated the ontogenetic gene expression patterns of the mouse nrp gene coding for Neural Regeneration Protein (nrp gene with GenBank identification RefSeq: NM_001013372.2) in the developing pre- and postnatal mouse brain.
The mouse nrp gene has been found expressed in the embryonic cortical anlage specifically in neocortical layers that are coinciding with the day of emergence of the respective cortical layer during embryonic ontogenetic development. When looking for co-expressing factors for the nrp expressing cells, it becomes obvious that vimentin is the dominant molecule. This intermediate filament is expressed in the embryonic cortex solely in radial glial cells that are providing the scaffold for radial neural migration of excitatory neurons during neocortical development. A subgroup of mouse nrp expressing cells is co-localized with the neuronal specific protein MAP-2. This aforementioned immuno-cytochemical analysis provides indirect evidence that the NRP gene family is directly involved in “building” embryonic brain architecture by exerting a chemoattractive effect on migrating and developing neuroblasts. This process is followed by final neural differentiation by establishing a functional neural network. At day of birth (P0 mouse), nrp gene expression is ceasing in the cortical plate and emerges in the CA1/CA3 and sub granular zone of the hippocampal formation (active neuronal proliferation zone for neural precursor cells) as well as in the mitral/granular layer of the Bulbus olfactorius (Figure 1). The latter near “nasal” zone expression location could indicate that the nrp gene could well be the elusive “driver” of the rostral neural migration stream which originates from the anterior subventricular zone (SVZa) sending neuroblasts towards the B. olfactorius and replenishing and simultaneously renewing olfactory neurons during the lifetime of a mammal. It also has been recently established that the rostral migration stream is controlled by CXCR4 activation.4
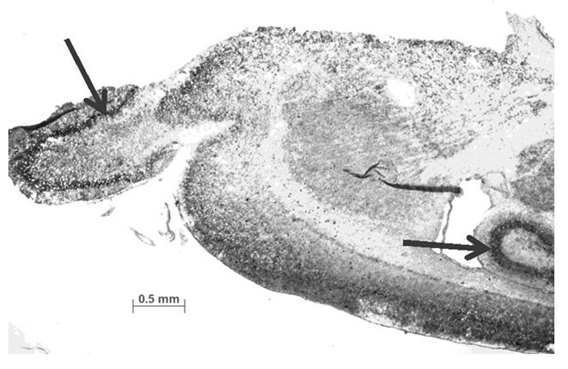
Figure 1 In situ hybridisation with nrp gene specific anti-sense cRNA probe when incubating it with sagittally processed P0 mouse brain slices is showing nrp expression in the olfactory mitral cell layer as well as hippocampal CA1/CA3 expression (arrows are indicating the nrp-positive regions). The cRNA probe is highly specific and results in no signal when being tested as sense probe. The probe consists of a nucleotide sequence (88-mer) deriving from the transcription start region of the mouse nrp gene.
This work has been performed by Gorba et al.1
Because of the extreme potency of the NRP peptides (characterized 24-mer mouse nrp-derived peptide shows efficacy with EC-50 values in the low femtomolar range within in vitro assay testing of neuronal survival and chemoattraction), this novel nrp gene and its encoded protein products is so far the most likely published candidate to drive the “construction” of neural sheaths in the mouse neocortex and the brain in general in a chemoattractive manner.1 In the human genome, we have annotated and experimentally verified at least four locations for nrp gene orthologues, namely 7q31.35 (human CAPS-2), 12q13.1 (human dermcidin), 13q13.2 (predicted human nrp gene, susceptibility locus for petite mal or absence epilepsy), 15q12 (predicted human nrp gene, the identified respective promoter region defines a locus with possible involvement in neocortical thickness variations). The drug development strategy is focused on fragments of the verified RefSeq gene sequence of the CAPS-2 protein because fragments of the dermcidin protein were much less potent than fragments of CAPS-2 in respect of providing neuroprotection and neuroregeneration when tested within relevant neuronal injury in vitro assays.
To the best of our knowledge, the smallest (shortest) possible amino acid sequence that provides complete neural regeneration and neuroprotection in the mammalian CNS derives from the vesicular human protein CAPS-2 (calcium-activator protein for secretion 2) and constitutes the region between amino acids 40-50 representing the length of 11 amino acids. CAPS-2 is a secretory vesicle priming protein with high expression in the cerebellum as well as lung, liver and testis.5,6 It is responsible for the secretion of BDNF and NT-3.7
CuroNZ’ drug development strategy involved the engineering of two changes into the respective 11-mer sequence (one alanine switch and one incorporation of the non-natural amino acid, alpha-aminoisobutyric acid instead of an alanine residue at position 9). This strategy provides improved physico-chemical shelf life stability for the compound. This drug candidate is named NRP2945 and is now ready for testing in healthy adult volunteers.
NRP2945 has shown a range of pharmacodynamics activities within different animal models of neurodegeneration including optic nerve ligation, MOG-EAE (Myelin Oligodendrocyte Glycoprotein induced Experimental autoimmune Encephalitis), hemi-section spinal cord injury and preclinical relevant models of epilepsy. NRP2945 completely suppressed pentylenetetrazol (PTZ) induced convulsive seizures in male adult Wistar rats.2 NRP2945 is also capable of suppressing typical absence seizures that are common to the rat strain GAERS when administered as a single subcutaneous bolus. We are currently testing chronic administration paradigms for seizure control in these rats. For the planned clinical phase I/II trial, children with severe refractory epilepsy being categorized as Lennox-Gastaut Syndrome (LGS) will be enrolled to monitor whether NRP2945 will succeed in significantly reducing the seizure load within these young patients who are experiencing a multitude of different types of seizures that are currently only partially controlled by approved adjunctive treatments.
In conclusion, in the future NRPs could provide a viable option for chronic treatment of neuro degenerative diseases by providing a high level of safety with no known off-target effects as well as long-lasting inhibitory network stimulating effects. NRP2945 is diminishing seizure activity during pathological states of global brain circuits. For example it is known that during Status Epilepticus, GABA A receptors are internalized into the cell cytoplasm and therefore are unavailable for extracellular administered and normally effective drugs like benzodiazepines. In contrast, NRP2945 can quickly upregulate protein expression of the synaptic GABA A receptor system as well as GABA and can therefore provide a meaningful inhibitory stimulus during severe states of epilepsy.
None.
The author declares no conflict of interest.

©2016 Sieg. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.