Journal of
eISSN: 2475-5540


Mini Review Volume 4 Issue 2
Department of Biomedical Engineering, Michigan Technological University, USA
Correspondence: Feng Zhao, Department of Biomedical Engineering, Michigan Technological University, 400 Townsend Drive, Houghton, MI 49931, USA, Tel 9064 8728 52, Fax 9064 8717 17
Received: March 26, 2018 | Published: April 19, 2018
Citation: Sharma D, Chica JF, Zhao F. Mesenchymal stem cells for pre-vascularization of engineered tissues. J Stem Cell Res Ther. 2018;4(2):41-43. DOI: 10.15406/jsrt.2018.04.00112
Engineered tissues with a thickness larger than 150μm require an embedded functional vascular network to support cell survival and integration with host vasculature after in vivo implantation. Mesenchymal stem cells (MSCs) can secrete trophic factors to promote angiogenesis and function as pericytes to stabilize endothelial cell engineered neo-capillary structures. Recently, studies have shown that co-culturing MSCs with endothelial cells (ECs) could develop a pre-capillary network in tissue scaffolds. To realize such an outcome, several factors need to be considered, including MSC source, cell seeding order, oxygen concentration, and extra cellular matrix features. The present mini review summarizes these crucial considerations and will provide beneficial references for successful development of functional pre-vascularized tissues.
Recent advances in the field of stem cell biology and tissue engineering have revolutionized therapeutic approaches to treat various diseases, especially chronic wounds, bone diseases, cardiovascular complications, and neurodegenerative diseases. Different stem cell types have been investigated for designing appropriate therapeutic treatments. Among them, approaches involving embryonic and induced pluripotent stem cells (iPSCs) are ethically and socially controversial. In addition, these stem cell types, due to their high pluripotency, contain risks of teratoma formation.1,2 In the past decade, mesenchymal stem cells (MSCs) have attracted considerable attention due to their straightforward and less invasive isolation procedure as well as their multi-differentiation potential. MSCs can differentiate into various cell types including osteoblasts, chondrocytes, adipocytes, smooth muscle like cells, endothelial like cells and cardiomyocyte like cells. Moreover, being immunoprivileged, allogenic MSCs encounter minimal risk of immune rejection. They also secrete various trophic factors, which can promote cell survival and tissue regeneration.3,4 These promising capabilities have made MSCs potential candidate for construction of various tissue-engineered products. Nevertheless, engineered tissues with a thickness larger than 150μm require a functional micro vascular network to supply gases, nutrients, metabolic byproducts, and integrate with host vasculature after implantation.5 In the physiological capillary structure, endothelial cells (ECs) surround the vessel lumen. These ECs are themselves wrapped by pericytes, which stabilize the capillary structure.6 Numerous studies have confirmed that MSCs can function as pericytes.7,8 Consequently, in order to develop a capillary network in tissue scaffolds various research groups over past several years have investigated the outcome of MSC-EC co-cultures.9‒12 Compared with other pericyte candidates, MSCs are expected to play dual roles: stabilizing engineered micro vessels and performing their stem cell functions after implantation. In this mini review, we discuss important considerations for successful MSC-EC co-cultures to achieve a robust vascular network. These considerations include an appropriate cell source, cell-seeding order, optimum oxygen (O2) levels, appropriate extracellular matrix (ECM) and tissue scaffold features (Figure 1).
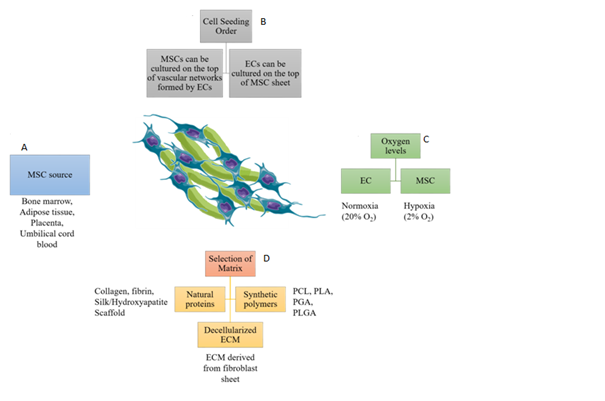
Figure 1 Considerations for MSC-EC co-culture for development of pre-vascularized engineered tissues. (A) Various sources from which MSCs can be isolated, (B) MSCs can be cultured on preformed vascular networks formed by ECs. In contrast, ECs cultured on MSC sheet forms better vascular networks, (C) MSCs maintain stemness and increase angiogenic growth factor secretion in a hypoxic environment. Whereas, ECs prefer normoxic environment for cell survival, proliferation and development of vascular networks, (D) Various natural and synthetic materials support MSC-EC co-culture. Decellularized ECM promotes development of robust vascular networks.
MSCs can be isolated from many sites in the body including bone marrow, adipose tissue, placenta, umbilical cord and umbilical cord blood.13 Each of these tissues provides a unique microenvironment, which might affect MSCs’ potential for vascular development during co-culture. Thus, it is crucial to compare MSCs derived from different tissue sources. Bone marrow derived MSCs are the most frequently used for biomedical applications. However, their isolation from bone marrow is associated with a low yield and high discomfort for patients.14 An alternative MSC source is adipose tissue, which is more accessible and provides a higher yield.15 Ma J et al.16 compared the angiogenic potential of bone marrow derived MSCs and adipose tissue derived MSCs by co-culturing them with human umbilical vein endothelial cells (HUVECs).16 Results from their animal study indicated that MSCs derived from these two sources have equal angiogenic potential.16 Besides bone marrow and adipose derived MSCs, placenta derived hMSCs showed a higher proliferation rate, multi-lineage differentiation capability and lower immunogenicity due to lack of human leukocyte antigen – antigen D Related (HLA-DR) gene fragment, which is a major histocompatibility complex (MHC) class II cell surface receptor.17 Similarly, umbilical cord blood derived MSCs have a high immunomodulatory effect due to lack of MHC-II expression. Thus, these MSCs can suppress the function of mature dendritic cells and proliferation of peripheral blood mononuclear cells.18 Despite these advantages, further studies are required to co-culture placenta and cord blood derived MSCs with ECs, as they can be easily isolated from these sources without relying on painful invasive methods.
Cell seeding order
In addition to the cell source, the selection of appropriate cell seeding order into the scaffolds is another crucial consideration for MSC-EC co-culture. During physiological angiogenesis, ECs first proliferate to develop proangiogenic cord like structures and then recruit pericytes to stabilize the newly formed vasculature.6 Similarly, during an in vitro experimental setup on a collagen–glycosaminoglycan scaffold, it was discovered that a delayed addition of MSCs on preformed EC networks during co-culture promoted vascular network formation relative to simultaneous seeding. EC-MSC co-seeding created cellular aggregation on the scaffold surface, which resulted in poor cell infiltration and immature vasculature formation.19 Moreover, physiologically larger MSCs also interfered with early self-assembly of EC networks. MSCs secrete vascular endothelial growth factor (VEGF), a critical growth factor that promotes angiogenesis. Addition of MSCs after 3 days of EC monoculture promoted VEGF expression, which further enhanced vascular network density compared to simultaneous addition of both cell types. The pre-vascularized functional construct showed anastomosis between engineered vessels and host vasculature upon sub-subcutaneous implantation in a rat model.19 In another study, an opposite cell-seeding order was employed to develop pre-vascularized sheets by co-culturing ECs on the top of a thick MSC layer.9 This pre-vascularized sheet was subsequently folded to form a 3D construct, in which ECs migrated to form a vascular network in both horizontal and vertical directions. The engineered vascular network anastomosed with host vasculature after subcutaneous implantation in immunodeficient mice.9 Similarly, we have cultured ECs on top of MSC layers to form a high-density vascular network.20 When comparing capillary network formation using these two opposite cell seeding approaches, the EC seeding on top of MSC sheets develops a highly dense and mature vascular network.
Oxygen levels
During MSC culture, provision of an appropriate O2 level maintains stemness and trophic factor secretion. MSCs present in bone marrow are exposed to a hypoxic microenvironment with 1- 6% O2 level, while vascular ECs experience higher O2 levels due to direct contact with O2 rich blood.21 To determine the effect of this physiological condition, we have examined the influence of physiological hypoxia (2% O2) on MSC stemness maintenance and angiogenic growth factor secretion relative to a normoxic condition (20% O2).22 Our results validated the beneficial effect of hypoxic preconditioning on hMSC’s ability to form an improved vasculature compared to normoxic preconditioning. However, the subsequent co-culture with ECs showed an opposite effect. The ECs cultured on top of the MSC sheet in a normoxic environment developed a longer, more extensively branched and uniform micro vascular network compared to their hypoxic counterpart.22 As mentioned above, ECs are generally exposed to higher O2 levels due to direct contact with blood. Thus, hypoxia might reduce their functional ability to construct capillary networks. We further implanted the prevascularized cell sheets, with combination of a split thickness skin graft, in a rat full thickness wound model. Results demonstrated that pre-vascularization of the MSC sheet significantly improved skin wound regeneration by alleviating graft contraction, preserving skin appendages, and inducing early neovascularization.20 These studies highlight the significance of O2 level during MSC and EC culture on the morphogenesis and function of engineered capillaries.
Selection of appropriate matrix or scaffold
Components of the ECM interact with cells and significantly affect their behavior. During early sprouting stages of angiogenesis in tissues, ECs break the underlying basal lamina and migrate toward connective tissue. Connective tissue contains a myriad of extracellular components including fibrillin, elastin, glycoproteins, and proteoglycans, but is extremely rich in collagen.23 Collagen I was found to activate the expression of Rho guanosine triphosphatases (GTPases) and sarcoma (Src) kinases, key regulators of actin cytoskeleton in micro vascular ECs, through the β1 integrin. Consequently, ECs cultured on collagen I exhibited precapillary cord like structure development due to disruption of vascular endothelial (VE)-cadherin.24 In sharp contrast, Rho GTPases and Src kinases were not activated when ECs were cultured on laminin-1, which prevented the development of precapillary cord like structures by inhibiting the VE-Cadherin disruption.24 Several other studies have confirmed the positive influence of collagen on capillary network formation during MSC-EC co-culture.16,19 Besides collagen, adipose derived and bone marrow derived stromal cells co-cultured with ECs in fibrin embedded spheroids also developed vascular network like structures.25 In order to mimic the native microenvironment more closely, we have developed decellularized ECM from human dermal fibroblast sheets.26 Importantly, these ECM sheets inherit the nano-fibrous structure of ECM and support robust vascular network formation during MSC-EC co-culture.26 As an alternative for natural ECM components, various biodegradable and biocompatible synthetic polymers can be used to develop scaffolds for co-cultures, such as polycaprolactone (PCL), poly(l-lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(lactic-co-glycolic) acid (PLGA).27 Although these synthetic materials are able to mimic the physical properties of ECM to some extent, they need to be further functionalized to create a more favorable environment for the cells. Combinations of synthetic polymers with naturally derived ECM can be used to fabricate an appropriate matrix for pre-vascularization approaches.
Current problems and future outlook
Although significant progress has been made in engineering micro vessels by optimizing MSC-EC co-cultures, challenges remain in engineering a mature micro vascular network with an open lumen, integrated vessel wall, and a physiological density and structure. Moreover, cell survival after in vivo implantation is another major concern. Studies have shown that MSCs experience a very low survival rate (< 5%) after in vivo implantation.28 Although MSCs are considered as immune-privileged due to the absence of MHC-II expression,29 in vivo testing showed that MSCs upregulate MHC-II expression at the inflammation site and can be recognized by the host immune system.30 All of these challenges should be considered when designing MSC therapies for future tissue engineering applications.
This study was supported by the National Institutes of Health (1R15CA202656) and the National Science Foundation (1703570).
The author declares that there is no conflict of interest.

©2018 Sharma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.