Journal of
eISSN: 2475-5540


Mini Review Volume 1 Issue 5
Department of Bioscience Technology and Center for Biomedical Technology, Chung Yuan Christian University, Taiwan
Correspondence: Guang Jer Wu, Department of Bioscience Technology and Center for Biomedical Technology, Chung Yuan Christian University, ChungLi, Taiwan, Tel +886 3265 3507, Fax +886 3265 3599
Received: March 08, 2016 | Published: November 4, 2016
Citation: Wu GJ. Human metcam/muc18 is a new diagnostic marker of and a driver for promoting and its specific sirnas, derived oligopeptides and antibodies be used for decreasing the malignant progression of prostate cancer. J Stem Cell Res Ther. 2016;1(5):201-203. DOI: 10.15406/jsrt.2016.01.00035
METCAM/MUC18, an integral membrane cell adhesion molecule (CAM) in the Ig-like gene superfamily, is not expressed in most normal prostate gland, or in all BPH, but overly expressed in most malignant prostate cancer. Its over-expression also correlates with the malignant progression of mouse prostatic adenocarcinoma in a TRAMP model, suggesting that it may be a diagnostic marker for malignant prostate cancer. To demonstrate this, three immunological methods are developed to show promises to use METCAM/MUC18 as a diagnostic marker for the presence of clinical prostate cancer. Enforced expression of METCAM/MUC18 in a human prostate cancer cell line, LNCaP, promotes tumorigenesis and initiates metastasis to multiple organs in a male nude mouse model, suggesting that it can promote the malignant progression of prostate cancer. ShRNAs in a lentivirus vector could decrease the tumorigenesis of another human prostate cancer cell line, DU145, in a male athymic nude mouse model. Taken together, METCAM/MUC18 is a new diagnostic marker of and a driver for promoting the malignant progression of and its specific siRNAs, oligo-peptides, and antibodies may be used for therapeutic treatments of clinical prostate cancer.
Keywords: METCAM/MUC18, cell adhesion molecule, immunoglobulin-like gene super family, biomarker & driver, prostate cancer malignant progression, animal models, siRNA blocking, clinical therapy
BPH, benign prostatic hyperplasia; PIN, prostatic intracellular neoplasia; ELISA, enzyme-linked immunosorbent assay; LFIA, lateral flow immunoassay
Prostatic carcinoma is the most frequently diagnosed cancer and the second most common cause of cancer death in American males.1 Most prostatic carcinomas are localized within prostate gland and have no obvious symptom; thus no treatment is required. However, prostatic carcinoma in some patients becomes malignant and and they often succumb to death. Unfortunately, how localized tumors become aggressive cancers are not understood.2 Furthermore, in clinical practice a precise prognosis of the carcinoma in any incidence is not easy because most prostatic carcinomas are multi-focal, the pathological grade observed in needle biopsies do not often represent the entirety of the malignant potential of the tumor.3 Moreover, an increased serum PSA level, which is commonly used in diagnosis for the presence of malignant prostatic carcinoma, has a false diagnosis rate of 20-25% and does not always reflect a pathological grade or the presence of a malignant cancer. This is because PSA, a serine protease, is not specific for the cancer, though it is only expressed in a prostate gland.4 Many possible diagnostic markers for malignant prostatic carcinoma cancer have been developed, however, none of them have been proven valid in phase III clinical trials;5 most of them are not capable of accurately differentiating fatal aggressive cancers from (not fatal) indolent ones.6 As such, there is still an urgent need to search for a better diagnostic marker for the early detection of the malignant potential of prostatic carcinomas. Ideally, it would be better to find a diagnostic molecular marker that also plays a key role in converting an indolent cancer into an aggressive cancer. After being identified, the marker may also be used for designing an efficacious treatment for the aggressive cancers. As shown below, METCAM/MUC18 may fulfill this need.
Human METCAM/MUC18, an integral membrane cell adhesion molecule (CAM) in the immunoglobulin-like gene superfamily, can perform typical functions of CAMs, such as cell-cell and cell-extracellular interactions, crosstalk with intracellular signaling pathways, and modulating social behaviors.7–8 The protein structure of METCAM/MUC18 is depicted in Figure 1.
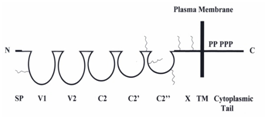
Figure 1 HuMETCAM/MUC18protein structure. SP stands for signal peptide sequence, V1, V2, C2, C2’, C2’’ for five Ig-like domains (each held by a disulfide bond) and X for one domain (without any disulfide bond) in the extracellular region, and TM for transmembrane domain. P stands for five potential phosphorylation sites (one for PKA, three for PKC, and one for CK2) in the cytoplasmic tail. The six conserved N-glycosylation sites are shown as wiggled lines in the extracellular domains of V1, between C2’ and C2”, C2’’, and X.
METCAM/MUC18 is not expressed in most normal prostatic epithelium, or in all of benign prostatic hyperplasia (BPH), but is expressed in most prostatic intracellular neoplasia (PIN), high grade prostatic carcinomas, and metastatic lesions.9–10 The expression of METCAM/MUC18 is also parallel to the malignant progression of mouse prostate adenocarcinoma in a transgenic model, TRAMP.11 Since there is a positive correlation of over-expression of METCAM/MUC18 with the pathological grade of clinical prostatic carcinoma and with that of the mouse adenocarcinoma in a transgenic mouse model, TRAMP, I suggest that METCAM/MUC18 is a possible new diagnostic marker for the malignant potential of prostatic carcinoma.12 Recently we further used and developed immunological methods, such as immunoblot (western blot) assay, enzyme-linked immunosorbent assay (ELISA),13,14 and the more simplified gold nanoparticles-based lateral flow immunoassay (LFIA)15,16 to show that the serum concentration of METCAM/MUC18 is directly proportional to the serum PSA level. Furthermore, the serum concentration of METCAM/MUC18 is significantly higher than that in normal individuals, suggesting that METCAM/MUC18 has a high possibility to serve as a new diagnostic marker for the detection of prostate cancer, as shown in Figure 2. As such, METCAM/MUC18 has a high probability to, at least, complement, or perhaps substitute, the PSA test for the diagnosis of the malignant potential of prostatic carcinomas after further improvement of the test.

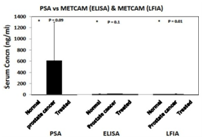
Figure 2 HuMETCAM/MUC18 may be used as a biomarker for the prediction of the malignant potential ofprostate cancer. The top panel shows the serum METCAM/MUC18 concentrations determined by gold nano-particles-based LFIA in comparison with ELISA. The bottom panel shows the serum METCAM/MUC18 concentrations determined by gold nano-particles-based LFIA and ELISA in comparison with PSA test.
METCAM/MUC18 can promote the malignant progression of prostatic carcinoma. Furthermore, enforced expression of METCAM/MUC18 in a human prostate cancer cell line, LNCaP, augments epithelial-to-mesenchymal transition (in vitro motility and in vitro invasiveness) and promotes in vivo tumorigenesis and initiates its spreading to many organs after injection of the cells in the prostate gland in male nude mice, suggesting that METCAM/MUC18 is truly a metastasis gene and can promote the malignant progression of human prostate cancer cells.17–21 From our mechanistic studies, METCAM/MUC18 may modulate these processes by augmenting proliferation, increasing the AKT-signaling pathway, boosting up aerobic glycolysis and increasing angiogenesis of prostate cancer cells; however it has no effect on apoptosis.17–19
METCAM/MUC18-specifc siRNA may be used for therapeutic blocking of malignant progression of prostate cancer. Three shRNAs, which are expressed in a lentivirus vector, have been demonstrated to decrease the tumorigenesis of another human prostate cancer cell line, DU145, which endogenously expresses a high level of METCAM/MUC18, in Balb/C athymic nude male mice,22 suggesting that shRNAs in a lentivirus vector may be used for therapeutic treatment to arrest the malignant progression of clinic prostatic carcinoma, as shown in Figure 3.

Figure 3 Tumorigenicity of METCAM/MUC18-specific shRNAs transfected DU145 clones. METCAM/MUC18-specific shRNA #72, 24, and 27 in a lentivirus vector blocks tumorigenesis of DU145 cells. ShRNA 46 is a non-METCAM/MUC18 shRNA control.
Moreover, soluble METCAM/MUC18 could block angiogenesis of LNCaP tumors in a pre-clinic athymic nude mouse model.23 Based on the above evidence, METCAM/MUC18 may have a high potential to serve as a new diagnostic marker to detect the emergence of clinical prostatic carcinoma. METCAM/MUC18-specifc shRNAs, METCAM/MUC18-derived oligo-peptides, and humanized anti-METCAM/MUC18 antibodies23–25 may be used as therapeutic means to arrest the malignant progression of clinical prostatic carcinoma.
I thank financial supports from Emory University School of Medicine (USA), Chung Yuan Christian University, and grants from NSC (NSC-101-2320-B-033-001 and -003), Taiwan (GJW).
The author declares no conflict of interest.

©2016 Wu. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.