Journal of
eISSN: 2475-5540


Research Article Volume 1 Issue 6
Department of Pathology, University of Washington, USA
Correspondence: Stephen H Bartelmez, Department of Pathology, Beta Stem Therapeutics Inc, 2 Lower Crescent Ave, Suite 2, Sausalito CA 94965
Received: December 10, 2016 | Published: December 2, 2016
Citation: Sitnicka E, Storey C, Bartelmez SH. Functional resolution of long-term-and short-term-hematopoietic stem cells. J Stem Cell Res Ther. 2016;1(6):243-256. DOI: 10.15406/jsrt.2016.01.00044
Several studies have demonstrated that the hematopoietic stem cell (HSC) compartment consists of long-term repopulating (LTR) and short-term repopulating (STR) HSC. Here we describe an improved purification approach that identifies both LTR- and STR- HSC as being lineage negative, c-kit positive, with very low Hoechst 33342 retention (Lin-, c-kit+, Holow). However, further selection of cells based on their differential retention of Rhodamine 123 resolves cells into LTR-HSC and STR-HSC. We show that our sort method highly enriches for LTR-HSC (Rhlow) and STR-HSC (Rhhigh), and demonstrate that the Rhlow cells as single transplanted cells are able to engraft 70% of mice in a competitive long-term repopulating assay. We then describe several in vitro assays that resolve Rhlow and Rhhigh cells based on the ability of single cells to survive, form clones, vary the time to their first cell division, express a high proliferative potential (HPP) or to generate HPP daughter cells at the 2- to 8-cell stage. In the presence of IL-3 alone, single Rhlow cells divided rarely and then formed only small clones (~8 cells). In contrast, Rhhigh readily divided in IL-3 alone and went on to form large clones (~10,000 cells). However, in the presence of IL-3+IL-6+SCF, both cell populations cloned in vitro with high efficiency (>90%), although the proportion of HPP clones was significantly higher in the Rhlow cell fraction (~90% vs ~40%). Furthermore, In addition, we show that the time required by Rhlow cells to undergo their first cell division in vitro is relatively non-synchronous and longer than that of Rhhigh cells. In addition, an analysis of daughter cells generated during the initial cell divsions of Rhlow or Rhhigh cells in vitro showed that expansion or maintenance of total HPP daughter cells occurred only in the Rhlow cell fraction. We measured the proliferative potential of daughter cells derived from single Rhlow and Rhhigh cells at the 2-8 cell stage. At the 2-cell stage, Rhlow cells generated an increased number of HPP daughter cells (↑1.4-fold) compared to Rhhigh cells that appeared to maintain the total number of HPP daughter cells (1.0-fold). However, by the 8-cell stage, the total number of HPP daughter cells generated by Rhlow cells expanded to nearly double that of starting HPP numbers (↑1.9 fold), compared to a decline in total HPP daughter cells in 8-cell Rhhigh clones (↓0.5 fold). Our studies at the 2-cell stage directly demonstrate symmetrical divisions (2 HPP per 2 daughter cells) that result in HPP expansion. Thus, these studies of growth factor responsivness of purified HSC (survival, cloning efficiency, time to the first cell division) and differentiation pathways (their ability to generate HPP daughter cells) identify means to differentiate LTR- and STR HSC in vitro.
Keywords: hematopoietic stem cell, rhodamine, cloning efficiency, cell division, primitive core of stem cells
HSC, hematopoietic stem cell; LTR, long-term repopulating; STR, short-term repopulating; ACDU, automated cell delivery unit; CRU, competitive repopulating units
The hematopoietic system contains a primitive core of stem cells (HSC) that are characterized by having both an essentially unlimited lifespan and the ability to differentiate to provide all of the myelolymphoid lineages of the circulating population.1-5 The unlimited lifespan of the stem cell pool is accomplished through self-replication, while the differentiation out of the stem cell compartment results not only in lineage specific commitment and terminal differentiation but also clonal extinction.6 A number of groups have established methods to purify HSC using FACS selection methods based on either cell size, cell-surface antigens or lectins, or upon differential affinities for mitochondrial and DNA-binding dyes see reviews.7,8 The cells fractionated by these methods have been studied for their proliferative capacity, pluripotency and purity using such measurements as proliferative potential and differentiation in vivo and in vitro.
The most important of these tests is the ability to repopulate a lethally irradiated animal in all myelolymphoid lineages.9-17 These transplantation studies showed that the stem cell compartment is heterogeneous with respect to the lifespan of individual clones. Perhaps most striking was the demonstration of distinct HSC subpopulations whose ability to repopulate lethally irradiated mice ranged from short-term (weeks to months, STR-HSC) to long-term (> 6 months, LTR-HSC).7 Several groups have used different approaches to separate LTR-HSC and STR-HSC that resulted in different purities of the separated fractions. Jones et al.15 separated LTR-HSC and STR-HSC based on cell size for the first time using elutriation. Recently, Osawa et al.18 used monoclonal antibodies to specific stem cell antigens including CD34, and demonstrated that the CD34+ cell fraction was enriched for STR-HSC, whereas LTR-HSC were CD34 negative. Transplantation studies showed that single Lin-Sca-1+c-kit+CD34- cells provided long term repopulation in >20% of recipient mice.
Another approach to stem cell selection utilizes the fluorescence dyes Hoechst 33342 (Ho, an A-T base pair specific DNA-binding dye) and Rhodamine 123 (Rh, a mitochondrial membrane specific binding dye). Baines & Visser19 initially showed that the primitive hematopoietic cells retained low levels of Ho fluorescence. Neben et al.20 separated LTR-HSC and STR-HSC based on cell size and low levels of Ho fluorescence. Goodell et al.21 selected LTR-HSC based on low Ho fluorescence displayed simultaneously at two emission wavelengths. Several groups16,22-26 have also shown that Rh fluorescence can resolve LTR-HSC and STR-HSC. We previously published an enrichment technique that selects for HSC using lineage-negative marrow cells based on the sequential analysis of their Ho and Rh retention. This approach also effectively resolved LTR-HSC and STR-HSC based on differential Rh fluorescence.16 Our recent studies have focused on a modification of this enrichment procedure which we describe in detail in this study.
The aim of this study was two-fold: 1) to determine the repopulating ability of HSC purified by this modified approach using a competitive repopulation assay; and 2) to attempt to resolve LTR-HSC and STR-HSC in vitro.
Animals
Three- to six-month-old male congenic B6SJL CD45.1 and C57BL/6J CD45.2 mice were purchased from Jackson Laboratories (Bar Harbor ME).
Pre-fractionation of bone marrow
Mice were sacrificed, femurs and tibias were removed aseptically, and marrow was harvested by flushing with phosphate-buffered saline containing 2% fetal bovine serum (PBS/2% FBS). Low density cells were first isolated on a gradient by layering 5ml aliquots of 107 unfractionated cells/ml over 4.0ml Nycodenz (1.080g/ml, Nycomed, Oslo, Norway). Cells were centrifuged 20 minutes at x 400g, collected from the interface and washed with a 10-fold excess of PBS/2% FBS. Lineage committed cells were then removed by the following antibody-mediated magnetic bead depletion method. A double strength mixture of rat anti-mouse monoclonal antibodies (denoted Ab mix) was prepared as follows: anti-7/4, 4% vol./vol. (antigen expressed by neutrophils and activated macrophages, a gift from Professor Simon Gordon, Oxford, UK); anti-YW25.12.7, 10% vol./vol. (blasts and nucleated erythroid cells, a gift from Dr. Ivan Bertoncello, Peter MacCallum Cancer Institute, Melbourne, Australia); anti-Ter119, 4µg/ml (nucleated eryhtroid cells); anti-B220, 9µg/ml (B and pre-B lymphocytes); anti-CD4 and -CD8, 5µg/ml(T lymphocytes); anti-CD11b (Mac-1), 9µg/ml (granulocytes, macrophages); and anti-Gr-1, 11µg/ml (granulocytes, monocytes). The latter six antibodies were purchased from PharMingen (San Diego CA).
Briefly, the light density cells (<1.080g/cc) were resuspended in PBS/2%FBS at 10x106 cells/100µl of PBS, an equal volume of double strength Ab mix was added, and cells were incubated at 4˚C for 15 minutes. Cells were centrifuged through 2ml of FBS to separate cells from unbound antibodies, and were then transferred into 50ml centrifuge tubes (Corning, Corning NY) and rosetted with magnetic beads coated with sheep anti-rat IgG (Dynabeads M-450, Dynal, Inc., Great Neck NJ) using the following procedure: 10 times the number of magnetic beads in an equal volume was added drop wise to the cell suspension adjusted 350x106 cells/3.5 ml maximum per tube and mixed gently with a 5 ml pipette. The suspension was centrifuged at 20g for three minutes, then vigorously resuspended and transferred to a 15ml polypropylene tube. Then 4ml of PBS/10% FBS was added to reduce non-specific cell trapping into bead-cell aggregates, and the tube was placed into the Dynal magnet for three minutes. Cells not bound with the magnetic beads remained in suspension and were carefully removed with a pipette (this fraction was designated lineage-negative cells, [Lin-]).
Lin- cells were then counted, centrifuged and resuspended in 10µM Hoechst 33342 (Ho, Sigma Chemical, St Louis MO) in PBS with 10% FBS (adjusted to pH 7.2 with NaHCO3) and incubated at 37˚C for one hour. After 40 minutes of Ho incubation, Rhodamine 123 (Rh, Sigma) was added to the cell suspension (final concentration 0.1µg/ml; i.e., 20 minutes total incubation with Rh). After dye incubation, cells were centrifuged, chilled to 4˚C, washed once with PBS/2% FBS, labeled with phycoerythrin-conjugated anti-c-kit antibody (2B8 clone, PharMingen; 1µg/ml final concentration) for 10 minutes, then washed and resuspended at 0.75x106cells/ml in PBS/2% FBS. Finally, propidium iodide (Sigma) was added (2µg/ml final concentration) for detection of dead cells, and cells were analyzed by FACS immediately.
Fluorescence - activated cell sorting
The above pre-fractionated cells were analyzed and sorted on a FACStar Plus flow cytometer (Becton Dickinson, San Jose CA) equipped with dual argon lasers, and an automated cell delivery unit (ACDU). Cells were kept chilled at 4˚C with a recirculating water bath. Monochromatic light at 351-364nm and 488nm was used for Ho and Rh excitations, respectively. Forward light scatter was detected using 488bp10 and ND 1.0 filters. Ho emission was detected using a 515 lp filter in order to maximize signals from hematopoietic cells (22; Bartelmez et al.35 unpublished observations). Rh emission was detected using a 530bp20 filter, PE emission using a 575bp20 filter, and PI emission using a 610 lp filter.
Figure 1 shows sequential steps of the cell preparation and sorting. Cells were gated using the following steps: first, forward light scatter and PI fluorescence were analyzed, and viable cells (PI negative) were selected. Next, gates were further refined by selecting various percentages from the Rh fluorescence histogram: the lowest 10% (defined as Rh low) and the middle 40% of the peak (defined as Rhhigh). Then Rhlow and Rhhigh cells were analyzed for Ho fluorescence and c-kit receptor. Cells that simultaneously demonstrated low Ho fluorescence and expressed c-kit receptor were sorted as individual cells into 96-well plates or collected in bulk. These two sorted fractions were defined as: Lin-Holowc-kit+Rhlow and Lin-Holowc-kit+Rhhigh , respectively, henceforth designated Rhlow and Rhhigh Table 1 and Figure 2 show the antigenic characterization of the final sorted Rhlow and Rhhigh cells. .
Culture conditions
Single sorted cells were cultured in 96-well U-bottomed plates (Corning) in IMDM medium (Gibco BRL, Grand Island NY) with 12.5% fetal bovine serum (FBS), 12.5% horse serum (HS, Gibco), 2x10-5 M 2-mercaptoethanol (2-ME, Sigma), 10-7 M hydrocortisone (HC, Sigma) and antibiotics (penicillin/streptomycin, Gibco) supplemented with cytokines as described previously.27 Different lots of FBS and HS used in cultures were previously screened for their ability to support HPP colony formation of Lin- cells in agar. Serum-free medium formulated for hematopoietic cell support (QBSF-58) was purchased from Quality Biological, Gaithersburg, MD. This medium contains no 2-ME nor HC.
In all experiments cells were cultured for 1-21 days. U-bottomed plates were centrifuged after direct cell deposition and single cell verification was done by direct observation at 200X magnification at the center-bottom. Up to ~64 cells per clone could be counted accurately. At 14 days of culture the clone size was determined by counting using a hemacytometer. Where indicated, single cell survival was determined by trypan blue exclusion. Cell morphology was estimated on cytospins stained by the Giemsa-Wright method.
HPP-CFC and GM-CFC assays
Double-layer nutrient agar cultures consisting of a 1ml underlayer of 0.5% and 0.5ml overlay of 0.3% agar in 35mm Petri dishes were used as previously described.27 In the experiments where 2- and 8-cell clones were assayed, a 0.3ml under layer and 0.2ml of over layer were used. Saturating concentrations of cytokines (based on the maximum clonal proliferation assays) were added as indicated in the text: rrSCF (50ng/ml), rmIL-3 (100ng/ml), rhIL-6 (20ng/ml). Cultures were incubated at 37˚C in a 5% O2, 5% CO2, 90% N2 gas mixture. Colonies were counted at 14 days using a dissecting microscope at 10X magnification (for HPP colonies) or an inverted microscope at 25X magnification (for progenitor colonies). HPP colonies were identified as macroscopic colonies (>100,000 cells/colony) with diameters >1.0mm having a dense center. Progenitor colonies were identified as clonogenic groups consisting of 50 to <100,000 cells per colony.27 Clusters averaged ~20 cells per clone and commonly consisted of monocytes/macrophages.
Competitive repopulation was used to measure repopulation capacity of sorted cell populations. Either one, 10 or 20 Rhlow and Rhhigh cells were directly sorted into U-bottomed 96-well plates. The plates were centrifuged and one, 10 or 20 cells were directly counted using an inverted microscope. Wells either contained the expected number of cells or less, and wells with the desired cell number were marked and used for transplant. The assay was performed using a CD45.1/45.2 congenic mouse system. Recipient animals (C57BL/6J CD45.2) were exposed to a single 950cGy total body irradiation. 3x105 unfractionated (CD45.2) bone marrow cells were added to the wells containing the counted Rhlow and Rhhigh cells (B6SJL CD45.1) cells and were injected into the tail vein of the recipient. Six weeks to 10 months after transplantation the proportion of donor-derived (CD45.1) nucleated white cells in the recipient's peripheral blood was quantitated by FACS analysis. Peripheral blood was obtained by capillary puncture of the orbital venous plexus. Collected blood cells (100µl) were transferred into 1ml PBS/2% FBS, centrifuged for five minutes at x400g, resuspended in 100µl of PBS/2%FBS, and red blood cells were lysed with 1 ml of NH4Cl lysis buffer for 10 minutes at 37˚C. Then 2ml of PBS/2% FBS was added, cells were centrifugated for 10 minutes at x400g and washed twice with PBS/2%FBS. The nucleated cells were divided into two fractions and stained with fluorescein conjugated monoclonal antibodies specific against either CD45.1 antigen (A20 clone) or CD45.2 antigen (104 clone, PharMingen). After staining, cells were analyzed on an Epics Profile II (Coulter Electronics, Hialeah FL). Red cell contamination was eliminated by analyzing only CD45.1 and CD45.2 positive cells. Non-specific binding of anti-CD45.1 antibody was determined by binding to CD45.2 blood cells. The percentage of CD45.1 positive cells was calculated according to the formula:
The results from the transplantation assay were expressed as competitive repopulating units (CRU) per 105 and calculated according to the formula:
Sources of cytokine
Purified, recombinant growth factors were kindly and generously provided as follows: rat SCF from Dr. Krisztina M. Zsebo, Amgen Inc., Thousand Oaks CA; mouse IL-3 from Dr. Andrew Hapel, Australian National University (the activity of was 5 U per 1ng); human IL-6 (rhuIL-6) from Dr. Douglas Williams, Immunex Corp., Seattle WA; mouse IL-11 (rmIL-11) from Drs. Paul Schendel and Stanley Wolf, Genetics Institute, Boston MA. FLT3 ligand (FL) was purchased from PeproTech (Rocky Hill NJ). The concentrations of cytokines used in experiments are described in the Results section.
Repopulating ability of Rhlow and Rhhigh cells
In our previous studies we showed that selection of bone marrow cells based on the Lin-, Fwd/90o "blast cell window" and the low net fluorescence levels of Hoechst 33342 and Rhodamine 123 resulted in separation of the mouse stem cells into two transplantable cell populations: LTR-HSC in the Rhlow fraction and STR-HSC in the Rhhigh fraction.16 In order to increase recovery and reproducibility of the HSC isolation, all viable Lin- cells were selected as an initial sort step instead of a “blast cell” window. Further analysis of the Lin- Holow cells indicated that essentially all cells in this fraction expressed c-kit (data not shown), and therefore a c-kit selection step was incorporated into the Ho/Rh sort scheme. Using this modified sorting method, two final cell populations were selected: cells retaining low levels of Ho and low levels of Rh and expressing c-kit receptor (defined as Rhlow) and cells retaining low levels of Ho and high levels of Rh, still expressing c-kit receptor (defined as Rhhigh , see Figure 1). In order to establish repopulating abilities of cells selected by this modified sorting approach, 1-20 sorted CD45.1 cells from Rhlow or Rhhigh fractions were injected into irradiated recipients together with 3x105 support cells (unfractionated CD45.2 marrow). After 1.5 and 6-10 months post transplant, the recipient animals were analyzed for the presence of donor-type CD45.1 cells by FACS (Table 1). Unexpectedly, at 1.5 and 10 months, ~60% of mice exhibited donor repopulation after transplant of a single Rhlow cell, while 10 and 20 Rhlow cell transplants engrafted 100% of mice. The repopulation included T (Thy-1+), B (B220+) and myeloid cells (GR-1+ and/or Mac-1+) in all cases (data not shown). The repopulating ability and dynamics were also somewhat unexpected. Single Rhlow cells were able to provide a significant (but low) level of repopulation early after transplantation, and that level increased and was sustained over a 10-month period. As the number of Rhlow cells was increased to 20, both the initial and long-term repopulation became significant. Transplant of 10 or 20 Rhhigh cells also produced significant repopulation at the earlier time point, but unlike the Rhlow cells, the donor repopulation declined at the later time points as is consistent with a STR-HSC phenotype. In summary, the Rhlow cells exhibit a long-term (and short-term) repopulating ability, in contrast to Rhhigh cells that primarily exhibit short-term repopulating ability. Importantly, the ability of single Rhlow cells to tri-lineage engraft the majority of mice demonstrates not only their HSC characteristic, but also indicates the high purity obtained using this fractionation procedure.
Phenotype |
c-kit+ |
Sca-1+ |
Thy1.2 |
CD34 Neg |
H-2Db+ |
Holow Rhlow |
98.90% |
98.90% |
100% |
77.50% |
59.90% |
Table 1 The phenotype of Lin- Rhlow Holow Murine HSC
Lin- Holow Rhlow cells were stained with PE -labeled monoclonal antibodies against either: c-kit, Sca-1, CD34, CD45.1, Thy1.2 and H-2Db antigens and analyzed by FACS.
Data show percentage of positive cells in Holow Rhlow fraction. Data from a representative experiment.
In vitro comparison of Rhlow and Rhhigh cells
We analyzed cellular responses of the Rhlow and Rhhigh cells to diverse growth factor conditions by sorting these fractionated cells individually into 96-well (round-bottom) plates. All wells were verified and mapped for the presence of single cells. During 14 days of culture, cells were observed daily and cell viability, proportion of dividing cells, time to the first cell division and clone size were determined.
Cell survival in medium without growth factors
The viability of single cells was measured using trypan blue inclusion and light refraction under direct microscopic observation, and the ability to proliferate in response to growth factors. Clones derived from proliferating single cells were considered viable if more than 50% of cells in the clone were viable. In the absence of added growth factors, neither Rhlow nor Rhhigh cells divided. However, as shown in Figure 2, in serum-containing medium, 100% of Rhlow cells survived for the first 24 hours compared to only 63±3% of Rhhigh cells. After 48, 72, and 96 hours, 38±6%, 10±3%, 5±2% of Rhlow cells were viable compared to 14±2%, 3±3%, 0±0% of Rhhigh cells, respectively. However, in serum-free medium, the survival of Rhlow cells began to decline within the first 24 hours (89±6%). In contrast, after 24 hours the proportion of viable Rhhigh cells was the same as in medium with serum (65±18 vs 63±3%). After 48 hours in serum-free medium, the decline of live Rhlow cells compared to those found in serum-containing medium became more apparent (8±1% vs 38±6%). A similar proportion of Rhhigh cells survived for 48 hours in serum-free medium (17±2%) as with serum (14±2%). Neither cell type survived in serum-free medium beyond 72 hours. Cell survival in the presence of growth factors. Single Rhlow and Rhhigh cells were cultured for 14 days in medium with serum in the presence of IL-3, SCF or IL-6 alone or in combination. At the indicated time of culture, cell viability was measured. Figure 3 shows both viable single cells and surviving clones derived from dividing single cells (numbers in parenthesis indicate the proportion of cells that divided at least once). Among several single growth factors tested, only SCF and IL-3 significantly improved survival of both Rhlow and Rhhigh cells. In the Rhlow cell population, between days 4 and 14, SCF supported survival in 40% more cells than IL-3 (Figure 3 top pannel). In Rhhigh cells, either SCF or IL-3 increased cell survival to 100% during the first 24 hours. Thereafter, between days 2 and 14, SCF supported survival of 20% more Rhhigh cells than IL-3 (Figure 3 lower panel). IL-6 did not support survival of either Rhlow or Rhhigh cells (data not shown).
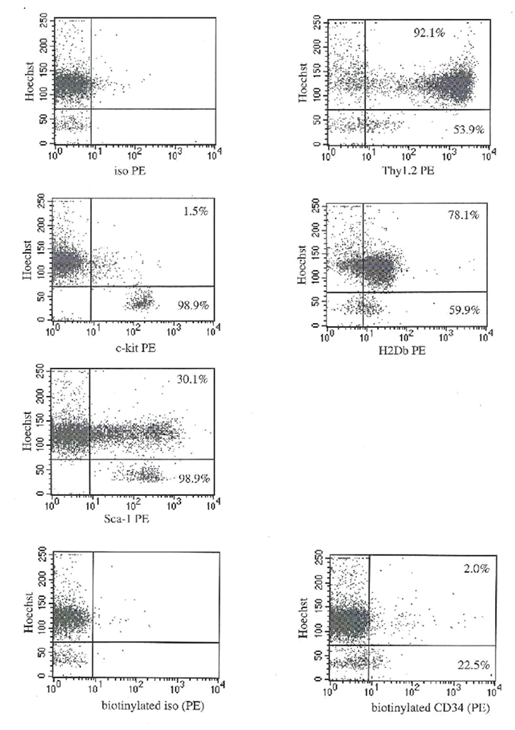
Figure 2 The cell surface phenotype of Lin- Holow Rhlow cells. Lin- Holow Rhlow cells were stained with PE-labeled monoclonal antibodies specific for either c-kit, Sca-1, Thy1.2, H2Db, or biotinylated monoclonal antibodies to CD34, and analyzed by FACS. Data from a representative experiment. Numbers represent percentage of positive cells within Holow subpopulation (post lin- and Rhlow selection). The lower box in each plot represents cells that were in G0, and upper box represents cells in G1/SG2/M phases of the cell cycle based on the degree of Hoechst 33342 retention
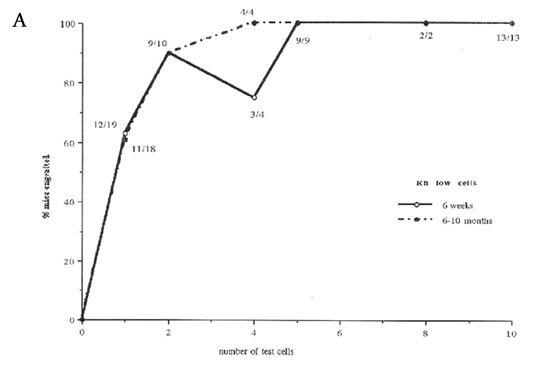
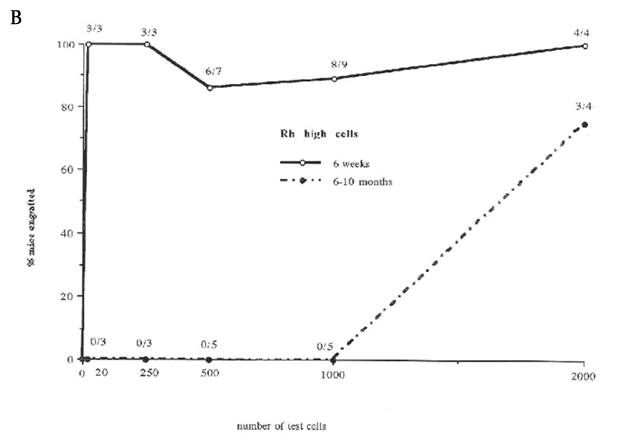
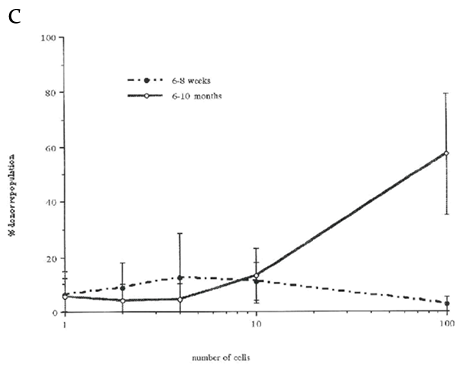
Figure 3 Percentage of mice repopulated after transplantation with lin-Holow Rhlow or Rhhigh cells (Dose range 1-2000 cells/ mouse)
Lethally irradiated recipient mice were injected with increasing numbers of Rhlowo r Rh h i g h c e l l s. At 6 weeks and 10 months post-transplantation, mice were analyzed for the % of donor type cells in peripheral blood using FACS. Results are shown as % donor cell repopulation and fractions indicate number positive mice within the test group. Mice were considered as positive when at least 0.5% of peripheral blood cells expressed donor type antigen (see Material and Methods). Data from 3 independent experiments where 2-20 mice were analyzed per group. A) Rhlow transplanted at 1, 2,4,8 and 10 cells per mouse. B) Rhhigh cells transplanted at 20, 250, 500, 1000 or 2000 cells per mouse. C) Rhlow cells transplanted at 1,2,4,10, 100 cells per mouse.
In the presence of multiple growth factor combinations, essentially all single cells and clones derived from single Rhlow high cells were viable until day 10 (Figure 3). After 14 days of culture in IL-3+IL-6+SCF, all the clones derived from single Rhlow cells contained >50% of viable cells (Figure 3 top pannel). In contrast, 35% of clones derived from single Rhhigh cells contained dead cells (mostly terminally differentiated granulocytes, Figure 3 lower panel or data not shown). Both Rhlow and Rhhigh cells required the presence of exogenous hematopoietic growth factors to survive in vitro for 14 days. However, without the addition of growth factors, Rhlow cells survived longer.
Proportion of single dividing cells (cloning efficiency)
To determine the proportion of cells that underwent at least one cell division (cloning efficiency), single Rhlow and Rhhigh cells were cultured for 14 days in the presence of single or multiple growth factor combinations. Among several single growth factors tested, only IL-3 alone and SCF induced one or more cell divisions (Table 2). In the presence of IL-3 alone, only 20±9% of Rhlow cells divided, compared to 78±8% of Rhhigh cells (Table 2). The average number of cells in the clones formed by Rhlow cells was 7±6, compared to 11089±8932 cells in the Rhhigh fraction (Table 2). In response to SCF alone, the same proportion of Rhlow and Rhhigh cells divided (65±13% and 63±30%, respectively; Table 2). The average cell number in the clones was 36±40 in the Rhlow fraction and 180±160 in the Rhhigh fraction. In the presence of two growth factor combinations, a similar proportion of Rhlow and Rhhigh cells divided (Table 2). In response to IL-3+IL-6+SCF, essentially all Rhlow and Rhhigh cells underwent at least one cell division (92±9% and 95±8%, respectively).
1.5 - 2 months |
Donor |
3 months |
6-10 months |
Donor |
19 |
(20/80) |
35.1 |
32.4 |
(8/92) |
16.9 |
(27/73) |
20.2 |
11.6 |
(2/98) |
1.2 |
(25/75) |
0 |
0 |
0 |
4.7 |
(71/29) |
4.2 |
0.5 |
(50/50) |
0.7 |
(0/100) |
0 |
0.8 |
(0/100) |
1.1 |
(39/61) |
3.1 |
1.1 |
(0/100) |
8.7 |
(54/46) |
2.6 |
0 |
0 |
4.9 |
(43/57) |
3 |
0.5 |
(50/50) |
5.1 |
(55/45) |
10.3 |
3.6 |
(0/100) |
0 |
0 |
3.6 |
0.6 |
(70/30) |
7.9 |
(86/14) |
10.7 |
died |
died |
5.4 |
(48/52) |
4.7 |
1.2 |
(3/97) |
0.3 |
(68/32) |
7.3 |
0.6 |
(100/0) |
0.5 |
(92/8) |
9.7 |
5.4 |
(55/45) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
12/19 = 63%* |
12/19 = 63% |
11/18 = 61% |
Table 2 Single LTR-HSC can Repopulate Lethally Irradiated Mice: Analysis of Individual Mice Transplanted with Single Lin- Rhlow Holow c-kit+ cells
% Donor Repopulation at 1.5-2, 3, and 6-10 months Post Transplant. Proportion of mice considered to be positive for CD 45.1+ donor cells =>0.5% in the peripheral blood. Single Rhlow Lin-Holow c- kit+ cells were deposited directly from the sorter into round bottom 96 well plates. Each well was verified for the presence of a single cell. Then, 200,000 CD 45.2+ support unfractionated marrow cells was added to each well with a verified single cell. Wells were harvested and cells were injected IV into lethally irradiated (950 rads) recipients. The presence of donor type cells was detected by two color FACS.
Time to the first cell division
To determine the time to their first cell division, single Rhlow and Rhhigh cells were cultured in the presence of different growth factors, cells were observed daily and the time when the first cell division occurred was noted. In the presence of IL-3+IL-6+SCF, cells in the Rhlow population did not divide within the first 24 hours of the culture, compared to 35±18% of Rhhigh cells (Figure 4 top panel). After 48 hours, only 24±13% of Rhlow cells underwent their first cell division, in contrast to 65±17% of Rhhigh cells. All Rhhigh cells completed their first cell division by 48 hours (Figure 4 top panel). The majority of Rhlow cells underwent their first cell division at day 3 (49±8% of cells) and day 4 (22±12%, Figure 4). A very small proportion of Rhlow cells (5±4%) did not divide until day 7 (Figure 4 top panel). In the presence of IL-3 or SCF alone, the time required to undergo the first cell division was significantly different than in the multiple growth factor combination. In IL-3 alone, the first cell division of Rhlow cel Rhlow very delayed, 67±20% of cells divided at day 14 of culture (Figure 4 lower panel). Similarly, in IL-3 alone Rhhigh cell division was significantly slower than in the presence of IL-3+IL-6+SCF, and their first cell division was not synchronized (Figure 4 lower panel). The time to the first cell division in the presence of SCF alone was similar to the response to IL-3+IL-6+SCF. In SCF alone, Rhlow cells started to divide at day 2 and a majority of cells completed their first division between days 3 and 7 (Figure 4 middle panel). However, 20% of single Rhlow cells did not divide until days 10-14 (Figure 4 middle panel). In contrast, 80% of Rhhigh cells completed their first cell division by day 2 (Figure 4 lower panel). A small proportion of Rhhigh cells underwent their first cell division in SCF between days 3-4 (Figure 4 middle panel). Regardless of the growth factor conditions, the time required for Rhlow cells to undergo their first cell division was longer and more heterogenous compared to Rhhigh cells (Figure 5 & Figure 6).
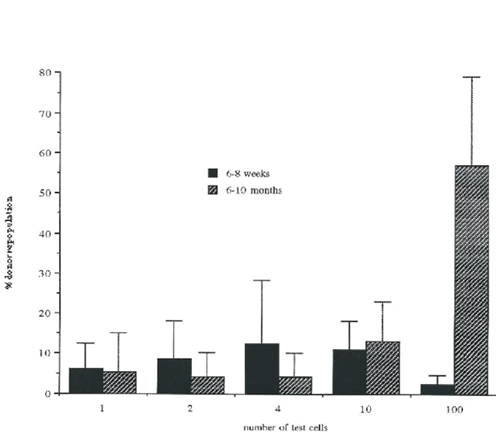
Figure 4 Percentage of donor repopulation after transplantation with different numbers of Rhlow cells.
Lethally irradiated recipient mice were injected with increasing numbers of lin- Rhlow Holow c-kit+cells (see Material and Methods). At 6-8 weeks and 6-10 months after transplantation, mice were analyzed for the presence of donor type cells in peripheral blood using flow cytometry. Results are shown as the level of donor repopulation. Data from 3 independent experiments where 2-20 mice were analyzed per group.
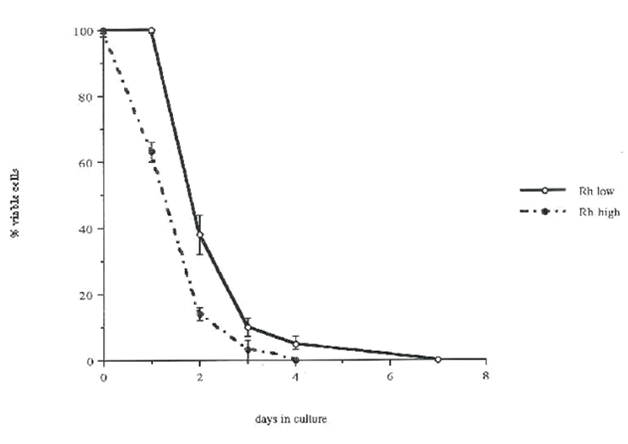
Figure 5 The comparison of cell survival in vitro in Rhlow and Rhhigh fractions.
Rhlow cells (solid line) and Rhhigh cells (broken line) were individually deposited from the sorter into 96-well plates containing serum-free medium alone ( Each day the cell viability was examined by trypan blue exclusion and the percentage of surviving cells was estimated. Data represent mean values ±SD from 3 independent experiments, in each experiment 30-50 clones were analyzed per group.
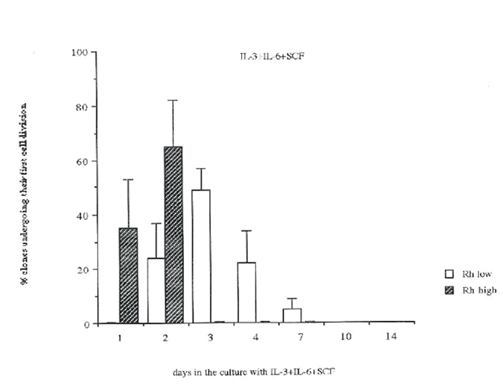
Figure 6 The comparison of the time required to undergo the first cell division in Rhlow and Rhhigh cell populations.
Rhlow cells (open bars) and Rhhigh cells (hatched bars) were individually deposited from the sorter into 96-well plates containing medium with IL-3+IL-6+SCF. Cells were observed under the inverted microscope daily, and the time of the first cell division was estimated. Data represents mean values ±SD from 3-10 independent experiments, in each experiment 50-80 clones were analyzed.
Proportion of cells with high proliferative potential (HPP)
To measure the proliferative potential, single Rhlow and Rhhigh cells were cultured for 14 days in 96-well plates in different growth factor combinations and the number of HPP clones was counted. HPP clones were not formed in either cell population in response to IL-3 alone, SCF alone, FL+IL-6, FL+IL-11 or SCF+FL (Table 2). In contrast,
in IL-3+IL-6+SCF a majority of Rhlow cells (88±8%) cloned with high proliferative potential compared to only 38±7% of Rhhigh cells. In the presence of SCF+IL3, SCF+IL-11, or SCF+IL-6, the number of HPP clones formed by Rhlow cells was lower than in IL-3+IL-6+SCF, however the proportion of HPP clones was always higher than in the Rhhigh cell population.
Daughter cell studies using multiple cell cultures
To estimate the proliferative potential of the daughter cells, sorted Rhlow and Rhhigh cells were allowed to divide under different culture conditions and then their daughter cells were assayed for HPP formation. In this first series of experiments, 100 Rhlow or 100 Rhhigh cells were cultured in the presence of SCF+FL, SCF+IL-6 or IL-3+IL-6+SCF, and after three, seven and 10 days of culture the total number of HPP generated and total cell production were measured. The data shown in Table 3 indicate a significant difference between these two cell populations. The highest number of HPP clones (2300±170) was generated from Rhlow cells after 10 days of culture in the presence of IL3+IL-6+SCF, compared to only 31±6 HPP generated from Rhhigh cells (Table 3). In the presence of SCF+IL-6 and SCF+FL after 10 days of culture, the number of HPP generated was lower than in IL-3+IL-6+SCF; however, more HPP clones were produced by Rhlow cells than Rhhigh cells (1600±300 vs 10±3 and 200±49 vs 58±20, respectively). The comparison between the number of HPP clones in Rhlow and Rhhigh cell populations at day 0 (67±10 and 24±1.5, respectively) and after 10 days of culture, indicated that a larger number of HPP clones were present in Rhlow cells, whereas the low level of HPP was maintained in Rhhigh cells. The total number of cells produced from 100 Rhlow cells and 100 Rhhigh cells after 10 days of culture in IL-3+IL-6+SCF, or SCF+IL-6 was very similar. In the presence of SCF+FL both cell populations proliferated slower and after 10 days 2400±720 cells were produced from Rhlow cells compare to 16500±8800 from Rhhigh cells.
Rhodamine Cut |
Growth Factors |
Cloning Efficiency1 |
% Clones >100,000 |
Average Number of |
low |
SCF,IL-3,-6 |
92±9 |
88±8 |
NA |
high |
SCF,IL-3,-6 |
95±8NS |
38±7* |
NA |
low |
SCF+IL-3 |
90±10 |
71±24 |
NA |
high |
SCF+IL-3 |
88 |
10 |
NA |
low |
SCF+IL-6 |
84±20 |
26±19 |
NA |
high |
SCF+IL-6 |
80±14NS |
5±4 |
NA |
low |
IL-3 |
20±9 |
0±0 |
7±6 |
high |
IL-3 |
72±8** |
0±0 |
11089±8932 |
low |
IL-6 |
0 |
0 |
0 |
high |
IL-6 |
0 |
0 |
0 |
low |
SCF |
65±13 |
0±0 |
36±40 |
high |
SCF |
63±20NS |
0±0 |
180±160 |
Table 3 Individual Lin-Holowc-kit+Rhlow and Rhhigh cells can be resolved by their proliferative response to SCF, IL-3 and/or IL-6
Rhlow and high cells were individually deposited from the cell sorter in 96- well-U-bottomed plates containing culture medium with growth factors: 10 ng/ml of IL-3/ml, 20 ng of IL-6/ml, 20 ng of IL-6, 50 ng of SCF/ml, 40 ng/ml of IL-3 alone, 50 ng/ml of SCF alone. Data represent values from 1 representative or 3-12 independent experiments, 30-80 clones in each group were analyzed +/- SD. After 14 days, clone size was analyzed. Proportion of single cells that divided at least once. Differences were analyzed using Student's t-test.1
*represents p<0.01, ** = p<0.001, NS = not significant.
Daughter cell studies using single cell cultures. To further study the mechanism of HPP production, the number of HPP generated from single Rhlow and Rhhigh cells at the 2- and 8-cell stage was measured. Single Rhlow and Rhhigh cells were directly deposited from the sorter into 96-well plates with 200µl of medium with IL-3+IL-6+SCF. Cells were observed daily, and at the 2- and 8-cell stage, clones were transferred to agar culture in the following manner: 150µl of medium was removed from the well, 200µl of 0.3% agar was added directly to the well and then transferred into previously prepared under layers containing growth factors and 0.6% agar. After 12-14 days the clone size was measured. The proliferative potential of daughter cells was analyzed in the following way. At the 2-cell stage, three possibilities can occur: neither of two daughter cells were HPP (0/2), one of the two daughter cells was HPP (1/2), or two of the two daughter cells were HPP (2/2). At the 8-cell stage, although more possibilities could be predicted, only three were analyzed: neither of the eight daughter cells were HPP (0/8), one of the eight daughter cells was HPP (1/8), or more than one of the eight daughter cells were HPP (>1/8). The loss of high proliferative potential is an indicator of daughter cell differentiation. The presence of two HPP daughter cells after the first cell division and more than one HPP daughter cell at the 8-cell stage indicates the generation of HPP. The total number of HPP formed at the 2-cell stage and 8-cell stage was calculated and compared to the total number of HPP before the first cell division. The results are shown in Table 4 & Table 5. As shown previously Table 2, in the presence of IL-3+IL-6+SCF, ~90% of Rhlow cells expressed proliferative potential, compared to ~40% of Rhhigh cells.
At the 2-cell stage, after the first cell division only 14% of Rhlow cells generated 0 HPP daughter cells compared to 69% of Rhhigh cells (Table 4). Among the Rhlow cells that generated HPP daughter cells after the first cell division, 33% of the cells produced 1 HPP daughter cell, and 53% of the cells gave rise to 2 HPP daughter cells. In contrast, among Rhhigh cells that generated HPP daughter cells after the first cell division at the 2-cell stage, an equal proportion of the cells gave rise to 1 HPP daughter cell and to 2 HPP daughter cells (17% and 14%, respectively; Table 4). It appears that differentiation already occurs after the first cell division in 69% of Rhhigh cells, compared to only 14% in Rhlow cells.
Days in Culture |
Rhodamine Cut |
Total Number of |
Total Number of |
% HPP-CFC |
0 |
high |
24±1.5 |
100±2 |
24% |
3 |
high |
50±10 |
4200±640 |
1.20% |
10 |
high |
31±6 |
143000±26000 |
0.02% |
0 |
low |
67±10 |
100±2 |
67% |
3 |
low |
180±17 |
198±17 |
91% |
10 |
low |
2300±170 |
168000±36000 |
1.40% |
Table 4 Lin-Holowc-kit+Rhlow and Rhhigh cells can be resolved by the number of HPP-CFC that are generated in the presence of SCF+IL-3+IL-6
One hundred Rhlow and Rhhigh cells were deposited from the cell sorter into 96-well-U-bottomed plates containing culture medium (see Materials & Methods) and 50 ng/ml SCF: 10 ng/ml IL-3/ml, and 20 ng/ml of IL-6. After 3 and 10 days in culture, cells were counted and plated in agar cultures using HPP growth conditions (see M&M). Data represent mean values from 2 independent experiments ± SD, 30-80 clones in each group were analyzed.
The next part of this analysis shows the proliferative potential of the daughter cells at the 8-cell stage, As illustrated by Table 4, 36% of Rhlow cells generated 0 HPP daughter cells compared to 88% of Rhhigh cells. Among the Rhlow cells that produced HPP daughter cells at the 8-cell stage, 17% of cells produced only 1 HPP daughter cell and 47% of the cells gave rise to more than 1 HPP daughter cells. In contrast, among the Rhhigh cells that produced HPP daughter cells at the 8-cell stage, an equal proportion of the cell gave rise to 1 HPP daughter cell or to more than 1 HPP daughter cell. It appears that 36% of Rhlow cells were differentiated at the 8-cell stage compared to 88% of Rhhigh cells. Also, 47% of Rhlow cells produced more than 1 HPP daughter cell compared to only 6% of Rhhigh cells.
The total number of HPP produced by Rhlow and Rhhigh cells at the 2-cell stage and the 8-cell stage appears to be significantly different (Table 5). In the Rhlow cell population at the 2-cell stage, 1.4±0.7 HPP were generated after the first cell division from 0.9±0.1 HPP. In the Rhhigh fraction, 0.4±0.1 HPP were generated from 0.4±0.7 HPP after the first cell division. At the 8-cell stage, 1.9±2.0 HPP daughter cells were generated from 0.9±0.1 HPP in the Rhlow population, compared to 0.2±0.5 HPP generated from 0.4±0.1 HPP in the Rhhigh population.
HSC type |
% HPP at |
0/2 HPP |
1/2 HPP |
2/2 HPP Daughter |
Average HPP |
Rhlow |
88±8 |
14% |
33% |
53% |
1.4±0.7 |
Rhhigh |
38±7 |
69% |
17% |
14% |
0.4±0.7 |
Table 5 Lin-Holowc-kit+Rhlow and Rhhigh cells can be resolved by the frequency of HPP daughter cells at the 2-cell stage
Fate of HSC Daughter Cells.
Rhlow and Rhhigh cells were individually deposited from the cell sorter into 96-well U-bottomed plates with culture medium (see Materials & Methods) containing IL-3+IL-6+SCF (10 ng/ml of IL-3/ml, 20 ng of IL-6/ml and 50 ng of SCF/ml). At 2-cell stage, clones were transferred into 24-well plates into agar cultures and assayed for HPP formation. Data represent values from 1 representative experiment; 30-80 clones in each group were analyzed.
The stem cell compartment has previously been shown to be heterogeneous. Based on in vivo assays, two distinct stem cell populations have been characterized by having either a long-term or a short-term repopulating capacity, presumably due to differences in their clonal extinction time. In recent years, several groups have established methods to physically separate LTR-HSC and STR-HSC from the marrow. These isolation methods were either based on cell size using elutration,15 or on the cell cycle stage and the ability to efflux the DNA-binding dye Hoechst 33342,19-21 or based on the degree of mitochondrial activation and efflux of the mitochondrial binding dye Rhodamine 123.16,22-26 In a previous study, we described a fractionation procedure based on the net fluorescence of both Hoechst 33342 and Rhodamine 123 that reproducibly separates and enriches LTR-HSC and STR-HSC.16 In this paper, we report a modification of this method that results in a higher enrichment and greater recovery of HSC, while maintaining good separation of LTR-HSC and STR-HSC. These modifications include: 1) the initial FACS selection of all viable Lin- cells (which increased HSC recovery) instead of a "blast cell" selection based on Fwd/90˚ light scatter; 2) selecting Rhlow and Rhhigh fluorescence cells prior to instead of after the low Ho selection (which increased HSC recovery); 3) adding c-kit as a positive selector;and 4) at a final step selecting HSC based on the bivariant plot, net Ho vs. c-kit expression (which clearly resolves the c-kit positive HSC population). It has been shown before that stem cells express c-kit on their cell surface.28,29
Competitive repopulation assays showed that Rhlow cells were highly enriched for LTR-HSC, in contrast to Rhhigh cells that provided little long-term repopulation. Moreover, the purity of the Rhlow fraction was remarkably high: ~60% of irradiated recipients were donor repopulated (11.9±9.6%) after transplantation of single cells. Other studies have demonstrated repopulation of single LTR-HSC, but not with efficiencies as high as we report in this study.19
Although LTR-HSC and STR-HSC are well defined in vivo, it has not been shown that these cell populations can be resolved in vitro (other than by phenotype immediately post-sort). Several studies reported that primitive hematopoietic stem cells required the presence of growth factors to survive in vitro.29-34 In particular, the role of SCF and IL-3 for stem cell survival in vitro has been well-defined.29-34 On the other hand, little is known about survival of individual stem cells in vitro in the absence of exogenous growth factors. In the presence of serum and absence of added growth factors, all single Rhlow cells survived for 24 hours, in contrast to ~60% of single Rhhigh cells. In serum-free conditions the survival of single Rhlow cells decreased, whereas survival of single Rhhigh cells was not affected.
Thus, it appears that a serum component(s) may mediate survival of a subpopulation of Rhlow but not Rhhigh cells. Miglliacio et al.33 reported that both Rhlow and Rhhigh cells died within 24 hours without the addition of exogenous growth factors, however these studies used a different fractionation procedure and multiple cell cultures. Interestingly, the proportion of Rhhigh cells that died within the first 24 hours of culture without the addition of growth factors correlated with the proportion of Rhhigh cells that underwent their first cell division at the time of culture when growth factors were added. Thus, cell cycle status and the probability of apoptotic death upon growth factor withdrawal may explain these observations.
Next, we found that Rhlow and Rhhigh cells could be resolved by their differential proliferative response to specific hematopoietic growth factors. The most striking difference occurred in response to IL-3 alone: the cloning efficiency and the clone size of Rhhigh cells was significantly greater than that of Rhlow cells. Li & Johnson26 and Miglliacio et al.40 also indicated that more cells responded to IL-3 alone in the Rhhigh fraction than in the Rhlow fraction. However, these studies were multiple cell cultures, and therefore the role of the cell-cell interactions could not be assessed. Several groups reported that both Rhlow and Rhhigh cells express similar levels of the α-chain of the IL-3 receptor.35-39 The higher response to IL-3 in Rhhigh population maybe due to the expression of the α-chain of the IL-3 receptor.
Our analysis of the time required by single Rhlow cells to undergo their first cell division showed marked differences between Rhlow and Rhhigh cells. Given that both Rhlow and Rhhigh cells were selected on the same low retention levels of Ho (i.e., apparent G0), this result was unexpected. On one hand this implicated Rh retention and mitochondrial inactivation as a mediator of the time required to the first cell division. However, Fleming et al.41 showed that 97% of Rhlow cells were in G0/G1 phase of the cell cycle, compared to 70% of Rhhigh cells. Similarly, Goodell et al.21 showed that only 1-3% of cells selected based on very low Ho retention were in the cell cycle. Migliaccio et al.33 demonstrated that colony formation of Rhhigh cells occurred between days 4 and 5 compared to day 21 for Rhlow cells.
The studies of Reddy et al.42 reported an estimated time of Rhlow cells to the first cell division to be 36-40 hours. We suspect that this difference may be due to the presence of IL-11 in addition to IL-3, IL-6, SCF and possible differences in the sorted cell population due to a different cell fractionation approach.
Although essentially all Rhlow and Rhhigh cells cloned with very high efficiency in the presence of IL-3+IL-6+SCF, their proliferative potential measured by the capacity to form macroclones was different. Li & Johnson26,32 demonstrated that both Rhlow and Rhhigh cells exhibited a high cloning efficiency and formed macroclones in response to multiple growth factor combinations. However, our results from single cell cultures showed that 90% of Rhlow cells respond to specific growth factors and form high proliferative potential clones in contrast to only 40% of Rhhigh cells. These results may indicate that the Rhhigh population contains more differentiated cells and correlate with the results from transplantation studies showing that cells retaining high levels of Rh are more differentiated then those that retain low Rh levels.23,24
Next, we analyzed the proliferative potential of daughter cells of Rhlow and Rhhigh cells. Significantly more HPP-CFC were generated from the same number of Rhlow than from Rhhigh cells regardless of the growth factors used. However, the total number of HPP daughter cells produced from the same number of sorted cells was markedly different in different growth factors (Table 3). The role of specific growth factors in the expansion of primitive hematopoietic stem cells have been studied intensively see reviews,1,2 in mice43-49 and human50-55 bone marrow.
However, Rhlow and Rhhigh cells have not been analyzed in these studies. In contrast to the above, total cell production in Rhlow and Rhhigh cultures was not different, except in SCF+Flt3 where total cell number was lower in Rhlow cells cultures (Table 3). Migliaccio et al.33 demonstrated that within five days of culture more cells were generated from Rhhigh cells as the result of shorter time required to enter the cell cycle. We also observed initially higher cell production in the Rhhigh population. However after 10 days of culture there was no significant difference in the cell number except in SCF+Flt3. The authors40 also showed that the proliferative potential of daughter cells measured by replating blast colonies declined after culture in the Rhhigh population.
To study the mechanism regulating the generation or maintenance of HPP daughter cells, we developed a new assay that allowed us to measure the proliferative potential of daughter cells derived from single cells. Given that >90% of Rhlow cells exhibited a HPP, we assumed that a daughter cell with an HPP might represent self-replication. As predicted from earlier studies, only Rhlow cells maintained or generated daughter cells with a high proliferative potential up to the 8-cell stage. After the first cell division in vitro, Rhlow cells more frequently generated two HPP daughter cells than did Rhhigh cells. In this manner the number of HPP daughter cells expanded, while asymmetrical first cell division that produced only one HPP daughter cell contributed to the HPP maintenance. The fact that Rhlow cells could undergo a “symmetrical” division at the 2-cell stage in vitro appears to correlate with their unlimited life span after transplantation. In a similar manner, the low number of HPP daughter cells generated from Rhhigh cells at the 8-cell stage may reflect their limited life span in vivo. Daughter cell transplants are in progress in an attempt to determine the repopulation ability of cells at these stages (Table 6).
Parameter |
Rhlow Cells |
Rhlow Cells |
Survival in vitro for 24 hrs without Growth Factors |
100±2% |
63±3% |
Cloning Efficiency+ in Response to IL-3 alone |
20±9% |
72±8% |
Clone Size in Response to IL-3 alone |
7±6 cells |
11089±9832 cells |
Time to the First Cell Division in IL-3, IL-6, SCF |
2-7 days |
1-2 days |
Clones with High Proliferative Potential (HPP) |
88±8% |
38±7% |
Total Number of HPP Generated from 100 Cells after 10 |
2300±170 |
31±6 |
Days in Culture IL-3, -6, SCF |
||
Proportion of 2/2 HPP Daughter Cells at 2 Cell Stage |
53% |
14% |
Average Number of HPP Generated after the First Cell |
1.4±0.7* |
0.4±0.7 |
Division (2-cell Stage)* |
Table 6 Summary: In vitro resolution of Lin-Holowc-kit+Rhlow and Rhhigh cells
+ cloning efficiency was defined as a proportion of cells that divided at least once in the presence of growth factors.
+daughter cells derived in the presence of IL-3+IL-6+SCF.
In summary, in this paper, we showed that LTR-HSC and STR-HSC could be resolved in vitro based on their growth factor/differentiation responses to specific hematopoietic growth factors in vitro.
Supported by RO1 grant DK48708, awarded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services.
The author declares no conflict of interest.

©2016 Sitnicka, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.