Journal of
eISSN: 2475-5540


Research Article Volume 1 Issue 6
1Department of Orthopaedic Surgery, Mubarak Al-Kbeer Hospital, Kuwait
2Department of Surgery, Kuwait University, Kuwait
Correspondence: Fathy G Khallaf, Consultant Orthopedic Surgeon, Department of Orthopaedic Surgery, Jahra Hospital, Ministry of Health, Kuwait, Tel +965 99160120, Fax 96524899617
Received: November 11, 2016 | Published: November 30, 2016
Citation: Khallaf FG, Kehinde EO. Estimation of mesenchymal stem cells (mscs) and monocytes in peripheral blood of acute traumatic head and spinal cord injuries in patients with concomitant diaphyseal femoral fractures: a clinical prospective controlled cohort study. J Stem Cell Res Ther. 2016;1(6):235-241. DOI: 10.15406/jsrt.2016.01.00043
There is some clinical evidence to suggest that fractures of long bones heal more rapidly in patients with severe head injury or acute traumatic spinal cord injury. The mechanism underlying this orthopedic phenomenon is not well understood. The aim of the current study was to investigate the bone healing of diaphyseal femoral fractures in patients with concomitant head or spinal cord injuries and to elucidate mechanism of a possible accelerated osteogenesis. The study recruited 52 group (A) patients with head injury, 50 group (B) patients with head injury and 58 femoral shaft fractures, 20 group (C) patients with spinal cord injuries, 21 group (D) patients with spinal cord injuries and 22 femoral shaft fractures, 60 group (E) patients with 69 femoral shaft fractures only, and 50 group (F) healthy subjects. All the femoral fractures in groups (B), (D), and (E) were treated by closed reduction and internal fixation by intramedullary nail. The fracture healing indicators were compared between patients of different groups and patients' blood samples were used to count circulating mesenchymal stem cells (MSCs) and monocytes using the flow cytometry. Results were analysed with statistical package of social sciences SPSS. Mean scores between two groups of patients were compared using chi square and the Student t-test. The study showed that femoral diaphyseal fractures in patients with head or spinal cord injury heal more expectedly, faster and with more callus formation and patients' blood samples showed statistically significant increase in circulating MSCs count and blood monocytes count in groups (A) to (D). The study revealed acceleration of femoral fractures in patients with concomitant head or spinal cord injuries and also demonstrated mobilization of distant bone marrow MSCs, homing early to fracture site and the role of monocytes in providing mediators to accelerate healing.
Keywords: mesenchymal stem cells, flow cytometry, callus formation, acute traumatic spinal cord injury, osteogenesis
Early clinical reports that researched the correlation between accelerated bone healing and acute traumatic nervous tissue damage in head or spinal cord injuries were inconclusive and demonstrated no evidence of accelerated union or increased callus formation.1-10
The primary objective of this prospective controlled study was to assess the effect of severe acute traumatic head injuries and spinal cord injuries on the healing of diaphyseal femoral fractures and the secondary objective was to test its effect on the count of circulating undifferentiated mesenchymal stem cells (MSCs) and monocytes in the patients' peripheral blood to elucidate the mechanism of bone healing in such patients.
Non- smoker patients in the age group of 18-60 years, and without history of chronic ill-health or systemic diseases were included in this study. Patients on permanent medications and therapy for diabetes mellitus, ischemic heart diseases, chronic renal failure, endocrine diseases, and patients on corticosteroids for bronchial asthma, rheumatoid arthritis, other inflammatory arthritis, and autoimmune diseases were excluded. The current study has obtained Kuwait University ethics committee approval, which included obtaining written consent from the patient or his next of kin if he is unconscious to participate in the study. From 19/09/2011, blood samples were collected from: 52 group (A) patients with severe head injury (defined as patients admitted to the ICU with GCS of 8 or less) without long bone fractures, 50 group (B) patients with severe head injury and diaphyseal femoral fractures, 20 group (C) patients with spinal cord injuries with complete quadriplegia or paraplegia without long bone fractures, 21 group (D) patients with spinal cord injuries with complete quadriplegia or paraplegia and diaphyseal femoral fractures, 60 group (E) patients with diaphyseal femoral fractures only, and 50 group (F) healthy subjects. The GCS was determined to assess the severity of head injury in groups (A) and (B). All femoral fractures in patients of group (B), (D), and (E) were treated surgically, by closed static reamed intra-medullary locking nail. The patients' biodata and characteristics of injuries of all groups were shown in Table 1.
Group |
0 hours |
24 hours |
One week |
2 week |
3 weeks |
A |
169.12 |
72.2 |
60.5 |
59.5 |
|
B |
1763 |
41.3 |
55.4 |
43.8 |
|
C |
204.334 |
54 |
50.5 |
30.5 |
|
D |
225.55 |
44 |
39.5 |
36 |
|
E |
40.76 |
55.3 |
112 |
81.5 |
|
*F |
13.21,2,3,4,5,6 |
Table 1 Human mesenchymal stem cells (CD105+ve/ CD14_-ve) in peripheral blood of patients with head injury with and without long bone fractures, spinal cord injury with and without long bone fractures, long bone fractures only and normal subjects
*Sample taken at time 0 (Control)
2p<0.0001, 3p<0.006, 4p<0.0001, 5p<0.004, 6p<0.0001
p2vs3<0.6, p3vs4<.04, p4vs5<0.05, P5vs6< 0.06
Radiological healing of fractures is defined by the presence of bridging callus, disappearance of fracture line or the continuity of cortex in at least in three of the four bone cortices appear in the antero-posterior and the lateral X-ray views, so a score of 3-4 points of basically bridging callus defines fracture union. We used CT to assess the maximal amount of union callus formed at the fracture site. Delayed union was defined as absence of radiological union criteria 3 months after the occurrence of the femoral fracture, while non-union was defined as no bridging callus and radiologically visible fracture line 6 months after the injury. The healing rate of femoral fracture was defined as the maximal thickness of union bridging callus in millimeters as observed in CT scan, divided by the time to healing in weeks. Time to radiological union, the maximal thickness of the amount of union callus formed, and healing rate of fractures were compared in three groups of patients: (B), (D), and (E).
Blood samples were withdrawn from the injured patients at: a) 24 hours, b) one week, c) two weeks, and d) three weeks from the time of injury and once from the healthy subjects. 10 ml of blood was withdrawn each time. The blood samples from different patients' groups were used to count the number of circulating mesenchymal stem cells and monocytes in peripheral blood using the flow cytometry, in each of the above groups of patients. Brief details of the method used are as follows: Flow Cytometry can be used for the analysis of peripheral blood cells, for obtaining the count of mesenchymal stem cells, and monocytes. This is done by using antibodies specific for these cell types. Specific staining of the leucocytes is performed by incubating the blood sample with the florescence conjugated antibody reagents. The red cells are then removed by lysis. The fluorescence and light scattering properties of the leukocytes are measured by flow cytometry.
The following fluorescent conjugated antibodies are used for this experiment:
Results were analysed with scientific package for social sciences (SPSS) for Windows (Version 16). Mean scores between the two groups of patients were compared using chi square and the Student t-test. p value < 0.05 was considered statistically significant.
The biodata of patients of all groups in the study were shown in Table 1. 52 patients have been recruited in group (A). The mean age in this group was 32.2, range (18-60) years. The patients were 49 (94.2%) males and 3 (5.8%) females. The findings of head CT scan in the patients of this group have been mentioned in Table 2.
Group |
0 hours |
24 hours |
One week |
2 week |
3 weeks |
A |
43.12 |
32.2 |
9.5 |
17 |
|
B |
29.93 |
17 |
24.6 |
30.5 |
|
C |
26.34 |
13.5 |
15.7 |
13.2 |
|
D |
27.55 |
24.5 |
26.3 |
22 |
|
E |
126 |
24 |
16 |
8 |
|
*F |
121,2,3,4,5,6 |
Table 2 Human mesenchymal stem cells (CD 90+ve/CD14-ve) in peripheral blood of patients with head injury with and without long bone fractures, spinal cord injury with and without long bone fractures, long bone fractures only and normal subjects
*Sample taken at time 0 (Control)
2p<0.0001, 3p<0.006, 4p<0.02, 5p<0.03, 6p<1
p2vs3<0.04, p3vs4<.0.52, p4vs5<0.05, P5vs6< 0.4
50 patients were included in group (B) and had a mean age of 31.4 (range 18-59) years. There were 48 (96%) males and 2 (4%) females in this group. The findings of head CT scan in the patients of this group have been mentioned in Table 2. Fifty eight closed diaphyseal femoral fractures were in the 50 patients in group (B), 36 (62.1%) of them were comminuted. These fractures were treated by closed reduction and internal fixation by static reamed interlocking intramedullary nail.
The mean time to union in this group was 6.9 (range 3-20) weeks. There were no cases of non-union of the diaphyseal femoral fractures in this group. The mean maximal thickness of union bridging callus was 26.3 (range 4-48) mm. The mean healing rate of fractures of long bones in this group of patients was 4.5 (range 0.2-10.6) mm/week, as shown in Figure 1 & Table 3.
Group |
0 hours |
24 hours |
One week |
2 weeks |
3 weeks |
A |
981.62 |
797.6 |
782.5 |
799.5 |
|
B |
712.53 |
727 |
978.6 |
912.17 |
|
C |
2086.34 |
1240 |
1040 |
1202.5 |
|
D |
879.55 |
860 |
870 |
858 |
|
E |
853.16 |
864 |
1089.5 |
545.5 |
|
*F |
512.561,2,3,4,5,6 |
Table 3 Monocytes in peripheral blood of patients with head injury with and without long bone fractures, spinal cord injury with and without long bone fractures, long bone fractures only and normal subjects
*Sample taken at time 0 (Control)
2p<0.0001, 3p<0.001, 4p<0.001, 5p<0.0004, 6p<0.002
p2vs3<0.5, p3vs4<0.4, p4vs5<0.4, P5vs6<0.3
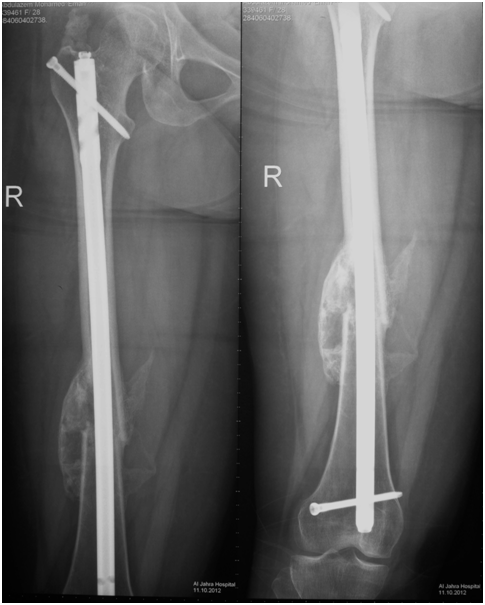
Figure 1 X-ray of femur with accelerated fracture healing and abundant callus formation in a group B patient with severe head injury and long bone fracture.
20 patients have been recruited in group (C). The mean age of the patients in this group was 32.7 range (18-49) years. These 20 patients included 16 (80%) males and 4 (20%) females. In all group (C) patients spine surgery procedures were undertaken. 21 patients were included in group (D) and had a mean age of 33.5 (range 22-48) years. There were 18 (85.7%) males and 3 (14.3%) females in this group.
22 closed diaphyseal femoral fractures were in the 21 patients in group (D), 11(50%) of them were comminuted. These fractures were treated by closed reduction and internal fixation by static reamed interlocking intramedullary. The mean time to union in of the diaphyseal femoral fractures in group D) was 6.2 (range 3-7.7) weeks. There were no cases of non-union of the femoral fractures in this group. The mean maximal thickness of union bridging callus was 29 (range10-48) mm. The mean healing rate was 4.7(range 2.6-7.5) mm/week, as shown in Figure 2 & Table 3.
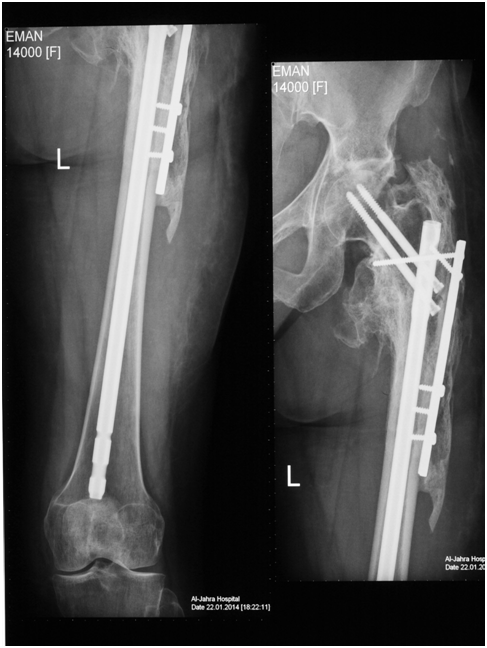
Figure 2 X-ray of accelerated union of fracture proximal third of the femoral diaphysis extending to the sub-trochanteric area with abundant callus formation in a group D patient with spine fracture-dislocation and paraplegia.
60 patients were included in group (E) and had a mean age of 33 (range 18-60) years. There were 55 (91.7%) males and 5 (8.3%) females in this group. 69 closed diaphyseal femoral fractures were in the 60 patients in group (E), 42(60.7%) of them were comminuted. These fractures were treated by internal fixation by static reamed interlocking intramedullary nail.
Among the 69 femoral fractures in the 60 patients in this group, 60 (87%) fractures united and 5 (7.3%) had delayed union, with union occurring 32 to 41weeks after the fractures occurred. Nine (13%) fractures ended up by atrophic nonunion and required secondary procedures, as shown in Figure 3. Two (2.9%) of these non-united femoral fractures developed metal failure of broken nails with the osseous failure of union. The mean healing time in this group of patients was 22.4 (range 13-41) weeks. The mean maximal thickness of callus in the united fractures in this group was 8.1(range 2-20) mm. The mean healing rate was 0.38 (range0.11 - 1) mm/week, as shown in Figure 3.
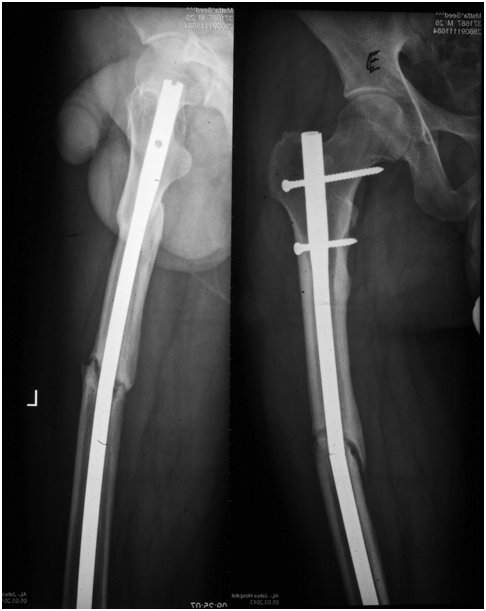
Figure 3 X-ray of fracture of the femur with atrophic nonunion and broken nail metal failure in a group E patient with long bone fracture only.
A comparative and statistical analysis of the 3 groups revealed that 50, 21 and 60 patients were recruited into groups B, D and E respectively. The 3 groups were comparable with respect to mean age. The mean (range) time to union of femoral fractures in groups B, D and E was 6.9 (3-20), 6.2 (3-7.7) and 22.4 (13-41) weeks respectively; (B or D versus E: p<0.001). The mean (range) thickness of callus formed at fracture sites of femoral fractures in groups B, D and E was 26 (4-48), 29 (10-48) and 8.1(2-20) mm respectively; (B or D versus E: p<0.001). The mean (range) healing rate of long bone fractures in groups B, D and E patients was 4.5 (0.2-10.6), 4.7 (2.6-7.5) and 0.38 (0.11- 1) mm/ week respectively; (B or D versus E: p<0.001), as shown in Table 3.
Measuring the cell count of mesenchymal stem cells (MSCs) (CD105+ve/ CD14-ve)and(CD 90+ve/ CD14-ve) in blood of patients from different groups showed high statistically significant cell count in patients with severe head injury with or without long bone fractures groups (A) and (B) and patients with spinal cord injuries with or without long bone fractures groups (C) and (D), especially in the first 24 hours post-injury which started to decline at the end of the first week, in comparison to the control groups of femoral fractures only group (E) and healthy subjects group (F) and although the count of MSCs was declined in the blood of patients of head injury with or without long bone fractures and patients of spinal cord injury with or without long bone fractures, it remained elevated statistically significant in comparison to its count in healthy subjects, as shown in Figure 4 & Figure 5 (P<0.001).
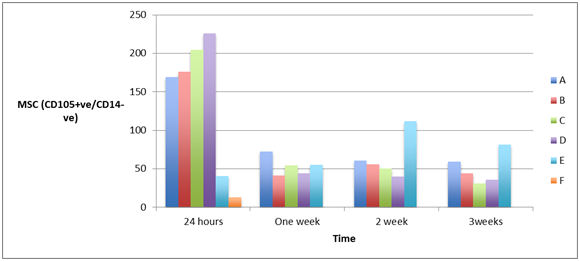
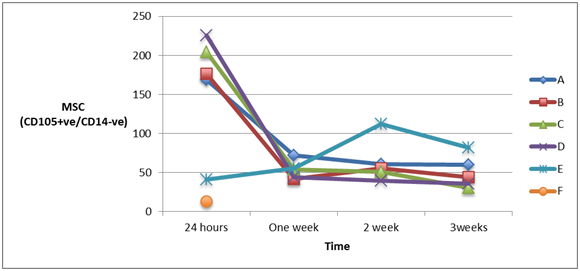
Figure 4A & 4B Human mesenchymal stem cells (CD105+ve/ CD14_-ve) in peripheral blood of patients with head injury with and without long bone fractures, spinal cord injury with and without long bone fractures, long bone fractures only and normal subjects.
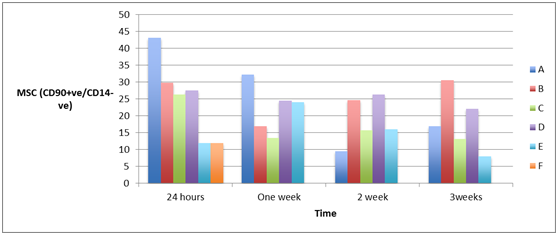
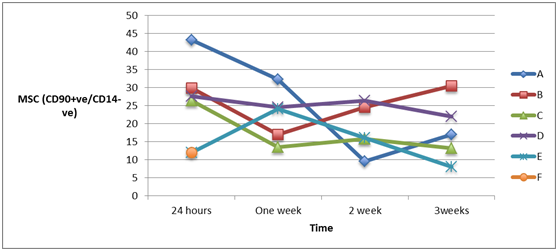
Figure 5A & 5B Human mesenchymal stem cells (CD 90+ve/ CD14_-ve) in peripheral blood of patients with head injury with and without long bone fractures, spinal cord injury with and without long bone fractures, long bone fractures only and normal subjects.
Measuring the cell count of monocytes (CD45–ECD) and (My4- FITC) in blood of patients from different groups showed high statistically significant cell count in patients of all groups in comparison to the monocytes cell count in healthy volunteers 24 hours post-injury (P<0.005). The highest increase was in patients with severe head injury only group (A) and spinal cord injury only group (C) patients (P<0.001). The cell count in all groups remained elevated statistically significant along the whole three weeks of follow-up, as shown in Figure 6 (P<0.005).
To our knowledge, the current study is the largest prospective controlled study that compared the time of union, amount of union callus, and rate of healing of 80 diaphyseal femoral fractures in 71 patients associated with severe central nerve tissue damage (58 fractures in 50 patients with associated severe head injury and 22 fractures in 21 patients with spinal cord injury) to 69 fractures in 60 patients without head or spinal cord injuries with matching of the variables of age, type of accident, type of fracture, the fractured long bone, associated injuries, and method of skeletal stabilization. Furthermore, in this study we excluded patients with chronic illness or systemic diseases, thus further reducing confounders that may affect fracture healing.
From the results of the two groups of patients with diaphyseal femoral fractures associated with head injury in group (B) and with spinal cord injury in group (D) compared to the group of patients with only femoral fractures group (E), we observed that fracture union occurred faster over a short period of time in groups (B) and (D) compared to group (E). Femoral fractures in group (B) and (D) patients united within mean of 6.6 (range 3-20) weeks compared to mean of 22.4 (range 13-41) weeks in group (E) patients, a statistically significant difference (p<0.001).
Another important finding of our study is that diaphyseal femoral fractures in head or spinal cord injury patients healed with more exuberant and florid callus formation compared with patients with femoral fractures only. The mean thickness of callus in groups (B) and (D) was 27.7 (range 4-48) mm, compared to 8.1 (range 2-20) mm in group (E) (p<0.001). Accordingly, the mean healing rate was also statistically significantly faster in groups (B) and (D) compared to group (E) {4.6 (range 0.2-10.6)mm/week versus 0.38 (range 0.11-1) mm/week}.
The study also showed that femoral fractures in patients with severe head injury or spinal cord injury all united and healed without a single case of nonunion or delayed union. However, 9 (13%) femoral fractures in group (E) patients had atrophic nonunion. Furthermore, 5 (7.2%) femoral fractures in group (E) had delayed union.
One of the points of weakness in this study was the determination of the exact time of union whether clinical or radiological and the controversies of definition of radiological union, that may require to be exact, to have daily X-ray which is impossible for very overt reasons. The study also, does not address the limitations in the analyses of "MSC" and "monocyte". The markers used to identify these cells are very limited. Furthermore, to be defined as an MSC the International Society for Cellular Therapy has proposed a set of basic requirements for a cell to be classified as a MSC: must be plastic culture adhesive cell with the ability to generate a colony-forming unit and differentiate into bone, cartilage, and adipose tissues. A similar concern is related to the limited set of markers used to define the "monocyte". All the possible measures and features have been taken to ensure that the definition of MSC and monocyte used for this study is rigorous enough.
Some articles in the literature were found to support the results of the current study. Newman et al.1 performed a retrospective study and demonstrated an unusually rapid healing of 13 closed long bone fractures in patients with concomitant severe head injuries.1 Giannoudis et al.2 performed a study that included 17 patients with head injury and associated femoral shaft fractures and 50 patients without head injury (25 treated with reamed and 25 with unreamed nailing technique), and these authors reported a significantly shorter mean time to fracture union in patients with head injury than either the reamed or the unreamed nailing groups without head injury.2 Yang et al.3 performed a retrospective study and compared the healing of femoral shaft fractures in 20 patients with associated traumatic brain injury (TBI) to 54 patients without brain injury, and these authors confirmed that an injury to the brain may be associated with accelerated healing and enhanced callus formation in femoral shaft fractures.3
To investigate the underlying mechanism of this accelerated osteogenesis, we counted the number of circulating mesenchymal stem cells and monocytes in peripheral blood using the flow cytometry, in each of the above groups of patients. The hypothesis was the possibility of mobilization of MSCs from distant bone marrow through systemic circulation that may home to the fracture site, and under the effect of growth factors and cytokines, may proliferate and differentiate into osteoblasts or chondrocytes, which accelerate fracture healing and in abundance callus.11-20
The results of our study to test this hypothesis showed a statistically significant increase in the count of undifferentiated mesenchymal stem cells (CD105+ve/ CD14_-ve MSCs and CD 90+ve/ CD14_-veMSCs) using fluorescent conjugated antibodies flow cytometry in patients with central nervous tissue damage whether severe head injury or spinal cord injury with paraplegia or quadriplegia with or without associated long bone diaphyseal femoral fractures, in comparison to their count in the peripheral blood of patients of control groups with femoral fractures only and healthy volunteers. This initial increase declined down by the end of first week till the end of the third and last week of flow-up (P<0.001). These results demonstrate mobilization of endogenous MSCs from distant bone marrow into systemic circulation and the drop in their count at the end of the first week may reflect the early homing and localization at the fracture site.
Afan et al.19 believed that the CNS has a role in the selective control of bone marrow cellularity that the denervation leads to decrease in the femoral bone marrow cellularity of undifferentiated mesenchymal stem cells and egress of abundance of these cells to the peripheral circulation.19
The elevated count of monocytes in the peripheral blood of all groups in comparison with the healthy subjects may demonstrate that Monocytes are progenitor cells that lead the inflammatory cascade reaction responsible for guiding revascularization and regeneration of bone at fracture sites. Monocytes seem to be the super cells that are involved throughout the process of bone healing.16,18
We conclude according to the results of this study that union of diaphyseal femoral fractures is ensured, augmented and accelerated in patients with concomitant acute post-traumatic head or spinal cord injuries which may be due to mobilization of endogenous MSCs from distant bone marrow to home early and in abundance to fracture site to secret cytokines and growth factors and differentiate into osteoblasts and chondrocytes to accelerate bone healing. We believe also that this mobilization could be explained by a neural or a combined neuro- humoral mechanism with a possible relative inhibition of the sympathetic nervous system.
This study article was a part of a project, which was funded "fully" by Kuwait Foundation for the Advancement of Sciences, KFAS under project code: 2010/1302/04.
The authors of this article are grateful to the sincere effort of Mr. Sherif Khallaf who made this piece of work possible.
The author declares no conflict of interest.

©2016 Khallaf, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.