Journal of
eISSN: 2475-5540


Research Article Volume 1 Issue 4
1Urology and Nephrology Center, Mansoura University, Egypt
2Mansoura University, Egypt
3Pathology Department, Mansoura University, Egypt
4Biochemistry Department, Zagazig University, Egypt
5Department of Biotechnology, Beni-Suef University, Egypt
Correspondence: Ahmed Nabil, Department of Biotechnology, Faculty of Postgraduate Studies for Advanced Sciences, Beni-Suef University, Beni-Suef, Egypt, Tel (+20)1000618349
Received: August 29, 2016 | Published: September 7, 2016
Citation: Zahran F, Nabil A, Karef AEl, et al. Effect of antioxidants and mesenchymal stem cells on cisplatin induced renal fibrosis in rats. J Stem Cell Res Ther. 2016;1(4):150-158. DOI: 10.15406/jsrt.2016.01.00026
Background: Mesenchymal stem cells (MSCs) have generated a great deal of excitement and promise as a potential source of all types of cells for cell-based therapeutic strategies. The reparative role of MSCs may be multifunctional and include the secretion of anti-inflammatory cytokines like TGFβ1 to limit apoptosis and dampen the inflammatory response. There are reports suggesting that antioxidants such as N N'-diphenyl-1, 4-phenylenediamine (DPPD) inhibit interstitial fibrosis induced by cisplatin. It inhibits lipid peroxidation and nephrotoxicity induced by cisplatin, where antioxidants make trapping for free radicals.
Aim: We aimed to investigate the inhibitory potential of either stem cells or DPPD on renal fibrosis in cisplatin induced tubulointerstitial fibrosis rat model.
Materials and methods: This study was carried on 40 male Sprague-Dawley rats (body weight 170-220g). Rats were divided into 4 groups as follow: Control group, received intravenous saline. Cisplatin group, received cisplatin (6mg/kg, i.p). DPPD group, received cisplatin (6mg/kg, i.p) at the start of experiments and three days after cisplatin administration, rats were given DPPD (0.5g/kg, i.p) every two days. MSCs group, received cisplatin (6mg/kg, i.p) at the start of experiments and three days after cisplatin administration, rats were given MSCs (1×106, i.v) single dose. 14 days after cisplatin (or saline) administration, blood samples were obtained and kidneys were removed for biochemical, histopathology and immunohistochemical markers investigations.
Results: In addition to the significant rise in urea and creatinine, cisplatin group showed atrophied glomeruli with tubular cells vacuolization and increased collagen deposition. Alpha smooth muscle actin (α-SMA) and fibroblast proliferation marker Ki-67 were found to be increased in renal tissue. Lipid peroxidation and collagen formation markers showed significant elevation. Both MSCs and antioxidant ameliorated cisplatin-induced nephrotoxicity to a great extent and showed marvelous anti-fibrotic effect as evidenced by histopathological, immunohistochemical and biochemical assessments.
Conclusion: Both MSCs and antioxidant (DPPD) were found to have potent potentials to inhibit tubulointerstitial fibrosis in cisplatin induced nephrotoxicity rat model.
Keywords: antioxidants, mesenchymal, stem cells, cisplatin, kidney, rat, fibrosis
Inflammation of the tubulointerstitial compartment, leading to fibrosis, is a major factor in the progressive loss of renal function. About 80% of total kidney volume is composed of tubular epithelial cells and cells within the interstitial space. There are also a small number of resident mononuclear cells and fibroblasts. It is widely recognized that progressive renal disease is accompanied by tubulointerstitial changes characterized by tubular atrophy, increased number of interstitial fibroblasts, phenotypic change of interstitial cells, accumulation of matrix proteins, and interstitial infiltrate of mononuclear cells.1
Deterioration of renal function is determined to a large extent by the degree of tubulointerstitial changes. However, the pathogenic mechanisms of tubulointerstitial changes have not yet been elucidated fully. Evidence from many studies suggests that common pathogenic mechanisms exist in the pathogenesis of tubulointerstitial changes. However, there is little detailed description of the molecular mechanism of renal fibrosis, and moreover, an effective treatment procedure has not been established.1
Cis-Diamminedichloroplatinum (II) (cisplatin) is an effective antitumor agent for testis, bladder, lung, and ovarian cancers. Its clinical use is limited due to the induction of tubular necrosis and acute kidney injury.2–4 In experimental animals, it causes renal interstitial fibrosis on the long term.5
The development of interstitial fibrosis is thought to cause irreversible renal dysfunction.6 Cisplatin increases the lipid peroxidation level in rat renal slices and the synthesis of hydrogen peroxide in cultured renal cells.6 These findings suggest that reactive oxygen species (ROS) have an important role in the pathogenesis of cisplatin-related fibrosis.7 Cisplatin induces cellular infiltration of macrophages and fibroblasts from the blood stream to the tubulointerstitium, where macrophages starts to secretes growth factors and cytokines that causes disequilibrium between apoptosis and proliferation of tubular cells leading to tubular atrophy.8 Also fibroblasts are activated into myofibroblasts that secrets collagen leading to further fibrosis.9
There are reports that antioxidants such as N N'-diphenyl-1, 4-phenylenediamine (DPPD) inhibit interstitial fibrosis induced by cisplatin.10,11 It prevents the increases in content of lipid peroxides and nephrotoxicity induced by cisplatin, where antioxidants are able to make trapping for free radicals..12
Also some of the most promising and frequent research in the field of regenerative medicine has focused on the use of stem cells. These cells, by definition, are undifferentiated cells with significant self-renewal capabilities. Additionally, stem cells are able to proliferate and establish daughter cell lines for tissue generation.13 The bone marrow is the source of mesenchymal SC (MSCs) from which many tissues may be obtained. The ability of adult MSCs to “transdifferentiate” could revolutionize regenerative medicine.14
MSCs are of great interest to both clinicians and researchers for their great potential to enhance tissue engineering. Their ease of isolation, manipulability and potential for differentiation are specifically what has made them so attractive.14 MSCs can repair the injured kidney through variable mechanisms where stem cells are able to make Homing to the injured kidney and secretion of anti-inflammatory cytokines like dampen the inflammatory response also it differentiate into tubular epithelial cells.15
This study aimed to study the effect of DPPD and mesenchymal stem cells on amelioration of renal fibrosis that induced by cisplatin in rats.
The study was carried on 40 male Sprague-Dawley rats (body weight 170-220g). Rats were bred and maintained in an air-conditioned animal house with specific pathogen free conditions, and were subjected to a 12:12-h daylight/darkness and allowed unlimited access to chow and water. All the ethical protocols for animal treatment were followed and supervised by the animal facilities, Medical Experimental Research Centre, Faculty of Medicine, Mansoura University. They were divided into 4 groups as follow:
At day14 after cisplatin or saline administration, blood samples were obtained and kidneys were removed.16
Mesenchymal stem cell dose injection
Mesenchymal stem cells were injected in rats through caudal veins 1×106 for each rat according to the protocol. Rats were killed under pentobarbital anesthesia 14 days after MSCs treatment.
Protocol of MSCs isolation
In this study we obtained cells from bone marrow of Sprague-Dawley rats.
Preparation of complete media: We used Dulbecco’s modified Eagles medium (DMEM) media so we aliquoting it under laminar air flow at 50ml tube each contain 45ml and preserve it at (2-8)°C. Fetal bovine serum was preserved at (-20)°C, then it was put in water bath at 37˚C until it was become liquid then it was deactivated at 56°C for 30min then it was aliquoted at 15ml tube each contain (10-15)ml. Anti-biotic preserved at (-20)°C so we put it at water bath for 20min then aliquoting at 15ml tube each contain (10-15)ml. At the 50ml tubes which contain 45ml DMEM we added5 ml FBS and 0.5ml anti biotic.
Methods of obtaining BM specimens for isolation and culture of BM-MSCs: The animals were killed by cervical dislocation, and then the skin was sterilized with 70% ethyl alcohol before cutting the skin. The femurs and tibia were carefully dissected from adherent soft tissues. Then they were placed into sterilized beaker containing 70% ethyl alcohol for 1-2min. The bones were put in Petri dish contain PBS for wash. The bones were taken to laminar air flow to extract the BM. The two ends of the bones were removed using sterile scissors. Bones were flushed with 3-5ml of complete media at one end, the marrow plugs were expelled from the opposite end of bone into sterile 15ml tube. The marrow plugs were cultured in 20ml complete media.
Culturing of bone marrow: The cells were cultured in 75cm2 tissue culture flask containing 10-15ml complete media in humidified incubator at 37°C in 5% CO2 and 95% air (by volume). The cultured cells were examined daily using the inverted microscope to follow up the growth of the cells. After 24h the old media were removed by aspiration using sterile pipette. The cells were then washed with 5ml PBS. Then 15ml complete media was added to the flask. MSCs were distinguished from other bone marrow cells by their ability to adhere to tissue culture polystyrene flask. The second exchange for media was done after 3-4 days. The media changed twice a week. The cells take 4 weeks to be confluent and be ready for Passaging.
Passage 1: Adherent cells attach themselves to surface of tissue culture polystyrene flasks or dishes using proteins secreted by the cells. To dislodge cells from the flask, the protein bridge must be broken. Trypsin is proteolytic (protein degrading) enzyme that will break proteins at specific places. EDTA is often found in Trypsin solutions. EDTA allows Trypsin to work more efficiently by engaging certain metal ions that may inhibit its activity.
The flask was examined under the inverted microscope to check the cell density and contamination. The old media was removed; the culture cells were washed with PBS. The PBS was left on the cells for at least 30 second to remove as much extracellular proteins as possible. The PBS was removed and 1-2ml of Trypsin/EDTA solution which has been prewarmed at room temp.
The flasks of cultured cells were sited inside the incubator for about for about 2min. Then the flasks were checked under inverted microscope to make sure that adherent cells were lifted off the flask. The detached cells appeared rounded. All remaining attached cells were excluded. 5ml of complete media was added to stop trypsinization, and then test the cell viability. Two new tissue culture flasks were prepared containing 15ml complete media. The 5ml in the old flask separated into the two flasks, and then incubated in humidified incubator at 37°C in 5% CO2 and 95% air.
Passage 2: Under the laminar airflow 14ml StemXvivo media was removed to gelatinized 75cm² polypropylene flask. The 2ml detached cells StemXvivo media suspension was transferred to the flask. The flask was incubated in 5% CO2 and 37°C After 3-4 days, the old media was removed containing the non-adherent cells. 5ml PBS 1x was pipette in the flask for wash then aspirated out of the flask. New 16 StemXvivo media 16ml StemXvivo media was added.
The process of feeding was repeated till adherent cells reach 80-90% confluence. On reaching 80-90% confluence, 4ml from the detaching solution was added (Collagenase 1a: 0.02%EDTA+0.5%BSA in PBS) (1:1) to detach the adherent cells. The flask was incubated at CO2 incubator 37°C and 5% CO2 for 4 minutes. After incubation, percussion on the bottom of the flask was done to detach the adherent cells.
Cell detachment was inspected under the inverted microscope. The detached cells were removed within the detaching solution out of the flask and pipette into sterile 50ml tube. 3ml DMEM media was added to the flask to collect more detached cells. The detached cells within the DMEM were removed from the flask and pipette into the sterile 50ml tube. Centrifugation at 300xg for 10min to get the cell pellet. Then supernatant was removed and the pellet was suspended in StemXvivo Media. The volume of StemXvivo Media was estimated according to the number of flasks needed for passage 2 (1ml for every 25cmm2 and 2ml for every 75cmm2). More passages were performed till the suitable number of cells was obtained.
Counting MSCs: Stem cells were suspended in 1ml of appropriate media. From this cell suspension, 10µl was removed for counting. Depending on the estimated cell number, a dilution factor between two and ten was used to count cells. Test the cell viability 10µl of cells was add to 10µl of Trypan blue 0.4% (Lonza, USA) and mixed them well and take 10µl of the mixture and put it on hemocytometer (Neubauer, Germany) and count cell under Ordinary microscope (Olympus CX31, USA).
Investigations provided to measure renal injury: Rats were sacrificed to evaluate the severity of injury in each kidney. At the end point all rats were sacrificed under anesthesia induced with phenobarbital sodium injection (50mg/kg body weight, intraperitoneal). Kidneys were removed, cut transversely into 3 slices. One part was embed in Tissue Tek (OCT compound) for immunofluorescent study, the second part was snap-frozen in liquid nitrogen for future genetic study and the last slice was fixed in 10% buffered formalin, and embedded in paraffin for morphological studies.17
Biochemical examination of blood and tissue: Blood samples were used for determination of serum creatinine and BUN. Tissue samples were used for determination of malondialdehyde (MDA) and hydroxyproline. These parameters were measured using an automated spectrophotometer (Slim Plus, Italy) and microplate Reader (Stat Fax 3200, Awareness).
Analysis of kidney histopathology: Serial 4-μm sections of the cortex and the medulla of the kidney was stained with hematoxylin and eosin (H&E) and Masson trichrome for pathological examination.
Indirect immunofluorescence was performed to detect collagen as a marker for tubulointerstitial injury. Also staining with α-SMA and Ki-67 immunohistochemistry markers to show the regeneration in renal tubulointerstitium and fibrosis was carried out.
Analysis of tubulointerstitial fibrosis
Total renal collagen was measured biochemically. In brief, an accurately weighed portion of the kidney was homogenized in distilled water, hydrolyzed in 10N HCl, and incubated at 110°C for 18h. The hydrolysate was dried by speed-vacuum centrifugation and redissolved in buffer (25g of citric acid, 6ml of glacial acetic acid, 60g of sodium acetate, and 17g of sodium hydroxide in 500ml.18 Total hydroxyproline in the hydrolysate was determined according to the chemical method of Kivirikko et al.19 Total collagen in the tissue was calculated on the assumption that collagen contains 12.7% hydroxyproline by weight. Final results were expressed as µg/mg kidney wet weight.19
Quantitative determination of MDA in tissue extract
MDA is the end product of fatty acid peroxidation reacts with thiobarbaturic acid in acidic medium at temperature of 95 Celsius and form a colored complex.20
Histopathological examination
Kidneys were perfused in a retrograde fashion through the abdominal aorta using saline 0.9% till complete clearance of the perfusion fluid, and then 10% neutral buffered formalin for in situ fixation. Both kidneys in all groups were harvested, cut longitudinally, and send for pathological evaluation in 10% neutral buffered formalin. Samples were processed and embedded in paraffin wax and sections (4μm thick) were evaluated for the following:
Tubular injury score: Sections were stained with H & E for light microscopic examination. In the cisplatin-induced ARF model, tubular injury is most evident in the outer stripe of the outer medulla (OSOM). Thus, the degree of proximal tubular injury in this area was assessed and quantified in accordance with the scoring system modified by Kinomura et al.21 Twenty randomly selected fields were observed at a magnification of x400. The degree of tubular injury was quantified as a score between 0 and 5 as the following: 0: normal; 1: tubular cells exhibiting desquamation from the tubular basement membrane (TBM), swelling, vacuolar degeneration and necrosis involving <20% of the tubules; 2: 20-40% tubules are involved; 3: 40-60%; 4: 60-80%; and 5: 80-100%.
Detection of interstitial fibrosis: Renal tissue samples of the experiment were stained with Masson trichrome. This stain is used as routine connective tissue stain. It stains collagen blue while nuclei are stained red to blue.22 To quantify interstitial fibrosis, the fibrotic area was measured by a color image analyzer (Image J 1.32) in five randomly selected fields in the OSOM and cortico-medullary junction. The blood vessels were avoided. The percentage of fibrotic area per unit area was calculated as per Yamate et al.23 1995 calculations.23
Immunohistochemical staining for α-SMA and Ki-67
Detection of α-SMA: The degree of α-SMA in all groups was assessed in renal tissue samples using a specific kit that detect α-SMA protein in rat cells (Dakocytomation, Glostrup, Denmark). The myofibroblast, a specialized fibroblast characterized by cytoplasmic stress fibers with α-SMA. The α-SMA is commonly used as a marker of myofibroblast formation. Antibodies against the α-SMA antigen have proven valuable. Quantification of α-SMA antigen is an indicator of myofibroblast and allows direct monitoring of myofibroblast in a specified tissue.24 The α-SMA in OSOM was counted in 20 randomly selected fields at 400x magnification in each kidney section.21
Detection of Ki-67 antigen: Ki-67 is a proliferation marker for well-developed myofibrblast which responsible for collagen secretion that cause fibrosis and detected in tissue samples using a specific kit that detect Ki-67 antigen in rat cells (Dakocytomation, Glostrup, Denmark).25 The Ki-67 antigen is a large nuclear protein preferentially expressed during all active phases of the cell cycle (G1, S, G2 and M phases), but absent in resting (G0) cells. Antibodies against the Ki-67 antigen have proven valuable. The number of Ki-67-positive nuclei in the OSOM was counted in 20 randomly selected fields at 400x magnification in each kidney section.21
Data were tabulated, coded then analyzed using the computer program SPSS (Statistical package for social science) version 17.0 to obtain descriptive data. Descriptive statistics were calculated in the form of mean±standard deviation (SD) and median, minimum and maximum. In the statistical comparison between the different groups, the significance of difference was tested using one of the following tests: ANOVA (analysis of variance): Used to compare between more than two groups of numerical (parametric) data followed by post-hoc tukey, Kruskal Wallis test: Used to compare between more than two groups of numerical (non-parametric) data followed by mann-whitney for pairwise comparisons or Repeated measures ANOVA (analysis of variance): Used to compare between more than two related groups of numerical (parametric) data followed by post-hoc LSD. A P value <0.05 was considered statistically significant.
The present study involved 4 groups and the results of studied parameters of these groups were analysed. At 14 days after cisplatin (or saline) administration, blood samples were obtained and kidneys were removed for biochemical, histopathology and immunohistopathological markers investigations.
Renal function monitoring
Serum creatinine and BUN in all studied groups: Renal function was monitored via assay of both serum creatinine and BUN. At day Table 1 shows that the control group had no significant change in serum creatinine and BUN. Meanwhile, only positive and antioxidant groups showed significant increase in serum creatinine and BUN compered to control group (p<0.001) but stem cells group showed no significant change compared to control group. While, antioxidant group showed significant decrease in serum creatinine and BUN compared to positive group (p<0.05) but stem cells group showed significant decrease compared to positive group (p<0.001) and significant decrease compared to antioxidant group (p<0.05).
Collagen formation and fibrosis markers assay
Hydroxyproline content in kidney tissue samples in all studied groups: Collagen formation and fibrosis were assessed via assay of hydroxproline content in renal tissue. At day 14, hydroxyproline content was found to be significantly increased in all studied groups compared to control group (p≤001). Meanwhile it was significantly decreased in antioxidant and stem cells groups compared to positive group (p≤0.001). Also, stem cells group showed significant decrease in hydroxyproline content compared to antioxidant group (p≤0.001) (Table 2).
Lipid Peroxidation assay
Malondialdehyde in tissue extract in all studied groups: In our study lipid peroxidation was monitored via assay of MDA content in renal tissue. According to (Table 3), MDA content at day 14 was significantly increased in positive compared to control group (p≤001). Significant increase in antioxidant group compared to control group (p≤0.05) was found. But compared to control group, stem cells group showed no significant change in MDA content. Moreover, antioxidant and stem cells groups showed significant decrease in MDA content compared to positive group (p≤0.001) with no significance found between stem cell group and antioxidant group.
Histopathological examination
Tubular injury score and fibrosis score: At day 14 kidneys were nephrectomised and were evaluated for tubular injury and fibrosis score (Figure 1). Both tubular injury and fibrosis scores were increased significantly in the three groups compared to control group. Meanwhile they were significantly lower in stem cells group and antioxidant group compared to positive group (p<0.05). It should be noted that stem cells group showed no significant change compared to antioxidant group (Table 4).
Immunohistochemical staining
Both α-SMA as a marker of myelofibrosis and Ki-67 as a marker for cell proliferation were assayed: At day 14, both α-SMA-positive cells and Ki-67 positive cells were significantly increased in all studied groups compared to control group (p≤001) but they showed significant decrease in antioxidant and stem cells groups compared to positive group (p≤0.001). Also, stem cells group showed significant decrease in α-SMA-positive cells compared to antioxidant group (p≤0.001) meanwhile it showed no significant change in Ki-67 proliferation marker compared to antioxidant group (Table 5 & Figure 2).
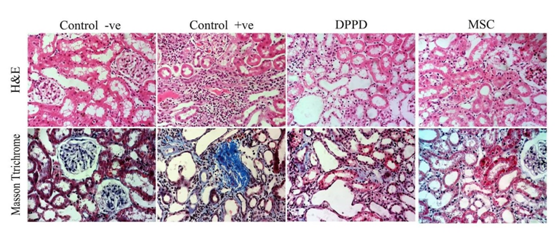
Figure 1 Effect of antioxidant and mesenchymal stem cell therapy on tubular injury and fibrosis score in rat fibrosis model induced by cisplatin.
Cr. (mg/dl) |
Control Group |
Positive Group |
Antioxidant Group |
Stem Cells Group |
|||
14 DAYS |
Mean |
0.49 |
1.08 |
0.85 |
0.66 |
<0.001** |
|
±SD |
0.03 |
0.2 |
0.13 |
0.18 |
|||
P1 |
<0.001** |
<0.001** |
0.06 |
||||
P2 |
0.008* |
<0.001** |
|||||
P3 |
0.04* |
||||||
BUN (mg/dl) |
|||||||
14 DAYS |
Mean |
19.18 |
54.1 |
34.16 |
26.45 |
<0.001** |
|
±SD |
1.49 |
6.56 |
6.37 |
6.04 |
|||
P1 |
<0.001** |
<0.001** |
0.028* |
||||
P2 |
<0.001** |
<0.001** |
|||||
P3 |
0.018* |
||||||
Table 1 Effect of antioxidant and mesenchymal stem cell therapy on serum creatinine and BUN in rat fibrosis model induced by cisplatin
P: Probability *: Significance ≤0.05 **: High Significance
Test used: One way ANOVA followed by post-hoc tukey for multiple comparisons
P1: Significance Relative to Control Group
P2: Significance Relative to Positive Group
P3: Significance Relative to Antioxidant Group
Hydroxyproline (ug/mg kidney tissue) |
Groups |
P |
||||
|---|---|---|---|---|---|---|
Control Group |
Positive Group |
Antioxidant Group |
Stem Cells Group |
|||
14 |
Mean |
21.44 |
71.92 |
37.72 |
30.06 |
<0.001** |
±SD |
0.48 |
2.89 |
2.36 |
1.44 |
||
P1 |
<0.001** |
<0.001** |
<0.001** |
|||
P2 |
<0.001** |
<0.001** |
||||
Table 2 Effect of antioxidant and mesenchymal stem cell therapy on Hydroxyproline in rat fibrosis model induced by cisplatin
P: Probability *: Significance ≤0.05 **: High Significance
Test used: One way ANOVA followed by post-hoc tukey for multiple comparisons
P1: Significance Relative to Control Group
P2: Significance Relative to Positive Group
P3: Significance Relative to Antioxidant Group
MDA (nmol /g tissue) |
Groups |
P |
||||
|---|---|---|---|---|---|---|
Control Group |
Positive Group |
Antioxidant Group |
Stem cells Group |
|||
14 |
Mean |
21.94 |
36.58 |
26.7 |
24.22 |
<0.001** |
±SD |
1.56 |
2.98 |
1.83 |
3.3 |
||
P1 |
<0.001** |
0.001* |
0.2 |
|||
P2 |
<0.001** |
<0.001** |
||||
P3 |
0.14 |
|||||
Table 3 Effect of antioxidant and mesenchymal stem cell therapy on MDA in tissue extract in rat fibrosis model induced by cisplatin
P: Probability *: Significance ≤0.05 **: High Significance
Test used: One way ANOVA followed by post-hoc tukey for multiple comparisons
P1: Significance Relative to Control Group
P2: Significance Relative to Positive Group
P3: Significance Relative to Antioxidant Group
Tubular Injury |
Groups |
P |
||||
|---|---|---|---|---|---|---|
Control Group |
Positive Group |
Antioxidant Group |
Stem Cells Group |
|||
14 DAYS
|
Median |
0 |
4 |
2 |
2 |
<0.001** |
Minimum |
0 |
2 |
1 |
1 |
||
Maximum |
0 |
5 |
3 |
3 |
||
P1 |
<0.001** |
0.001* |
0.005* |
|||
P2 |
0.01* |
0.004* |
||||
P3 |
0.68 |
|||||
Fibrosis score |
||||||
14 |
Median |
1.09 |
3.78 |
2.13 |
1.66 |
<0.001** |
Minimum |
0.95 |
2.36 |
1.35 |
1.12 |
||
Maximum |
1.35 |
5.03 |
2.6 |
2.21 |
||
P1 |
<0.001** |
0.001* |
0.025* |
|||
P2 |
0.02* |
0.001* |
||||
P3 |
0.28 |
|||||
Table 4 Effect of antioxidant and mesenchymal stem cell therapy on tubular injury and fibrosis score in rat fibrosis model induced by cisplatin
P: Probability *: Significance ≤0.05 **: High Significance
Test used: Kruskal wallis test followed by mann-whitney for multiple comparisons
P1: Significance Relative to Control Group
P2: Significance Relative to Positive Group
P3: Significance Relative to Antioxidant Group
α-SMA-positive cells/HPF |
Groups |
P |
||||||
|---|---|---|---|---|---|---|---|---|
Control group |
Positive group |
Antioxidant group |
Stem cells group |
|||||
14 DAYS |
Mean |
9.4 |
298.2 |
81.4 |
54.6 |
<0.001** |
||
±SD |
1.07 |
13.78 |
11.97 |
8.24 |
||||
P1 |
<0.001** |
<0.001** |
<0.001** |
|||||
P2 |
<0.001** |
<0.001** |
||||||
P3 |
<0.001** |
|||||||
Ki-67 positive cells/HPF |
||||||||
14 DAYS |
Mean |
7.9 |
83.9 |
55 |
53.8 |
<0.001** |
||
±SD |
2.02 |
16.08 |
6.22 |
5.45 |
||||
P1 |
<0.001** |
<0.001** |
<0.001** |
|||||
P2 |
<0.001** |
<0.001** |
||||||
P3 |
0.99 |
|||||||
Table 5 Effect of antioxidant and mesenchymal stem cell therapy on α-SMA-positive and Ki-67 positive cells/HPF in rat fibrosis model induced by cisplatin
P: Probability *: significance ≤0.05 **: high significance
Test used: One way ANOVA followed by post-hoc tukey for multiple comparisons
P1: significance relative to control group
P2: significance relative to positive group
P3: significance relative to Antioxidant group
Although the clinical management of AKI patients has significantly improved in recent years, we still lack specific therapies to enhance kidney repair. Recovery after acute injury is critical for patient morbidity and mortality in the hospital setting.26
Cisplatin is a potent antineoplastic drug used in the treatment of solid tumors. Clinicians should pay attention to its expected nephrotoxic effects. Many theories were reported about cisplatin-induced nephrotoxicity. These mechanisms included its role in induction of lipid peroxidation, mitochondrial dysfunction, injury of DNA, and structural protein degradation.27–29 Despite of that, the real mechanism is not well known so far. The platinum components of cisplatin bind to the DNA and this process leads to DNA damage and as a consequence of this reaction free radicals and reactive oxygen species will be elevated.30
Tubulointerstetial fibrosis is a directly related to cisplatin administration via the increased expression of reactive oxygen specie’s and the increased free radicals especially raised malondialdehyde levels as was previously reported.16,31,32
In this study we used cisplatin for induction of acute kidney injury in experimental rat model. Where we tried to study the potential impact mesenchymal stem cells and N N'-diphenyl-1, 4-phenylenediamine as member of the antioxidant agents in ameliorating the interstitial fibrosis induced via cisplatin. We thoroughly assessed the collagen formation and tubulointerstitial fibrosis on the histopathological, immunohistochemical staining and biochemical levels.
Our current study showed that the administration of either mesenchymal stem cells or the antioxidant (DPPD) offered significant amelioration of the nephrotoxicity and fibrosis induced by cisplatin. Where serum creatinine and BUN showed significant decrease in the stem cells and antioxidant groups in comparison to the cisplatin group as was also reported previously by other studies.33,16
Tubular injury and interstitial fibrosis are cardinal features of cisplatin induced nephrotoxicity on histopathological evaluation.34 Both of them had been carefully assessed and scored in our study. In line with some other reports.35,16 significant reduction has been found in both stem cell and antioxidant groups than in the cisplatin group and this was further proved and fortified via immunohistochemical assay of α-SMA and Ki-67 in renal tissue. Both α-SMA and Ki-67 contents were significantly lower in renal tissue denoting decreased myelofibrosis, collagen formation and cell proliferation in the stem cell group and antioxidant group more than cisplatin group.
Lipid peroxidation has been linked with cisplatin induced tubulointerstitial fibrosis as it has been suggested that binding of cisplatin to the renal base transport system and the following peroxidation of membrane lipids may account for its renal toxicity.36 In the present study, treatment with stem cells or antioxidant inhibited the increase in lipid peroxidation induced by cisplatin in renal tissue which was measured in terms of MDA, a stable metabolite of the free radical mediated lipid peroxidation cascade. MDA level was increased significantly in cisplatin treated group. Either stem cells or antioxidant has reversed the increased level of level to a considerable extent, thereby confirming their inhibition of lipid peroxidation in cisplatin induced acute kidney injury. The ability of either stem cells or antioxidant in inhibition of lipid peroxidation and their potent role as free radical scavengers was stated before in other reports.35,38
Also collagen synthesis and consequently fibrosis was much significantly suppressed as per hydroxylproline content assay in the renal tissue of the antioxidant and stem cell groups. Owing to the reports by Ognjanovic et al.37 and Ohnishi et al.38 they addressed the same findings as in our study.36,37,39
It is worthy to mention that in our study despite both MSCs and antioxidant (DPPD) proved to inhibit collagen formation and to have an anti-fibrotic effect but MSCs has been found to more potent.
We concluded that both mesenchymal stem cells and antioxidants had the ability to significantly ameliorate the collagen formation and interstitial fibrosis induced by cisplatin. May be more studies are still needed to solidify our findings and to study the other possible mechanisms underlying their anti-fibrotic potentials to get them from bench to bedside.
The authors would extend their thanks to the whole technicians in the Medical Experimental Research Centre, Faculty of Medicine, Mansoura University for their great dedicated work.
The author declares no conflict of interest.

©2016 Zahran, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.