Journal of
eISSN: 2475-5540


Short Communication Volume 4 Issue 3
1Centro de Ci
2Centro de Engenharia, Modelagem e Ci
Correspondence: Arnaldo R Santos, Universidade Federal do ABC, Centro de Ci, Tel 5511 2320 6233
Received: April 19, 2018 | Published: May 18, 2018
Citation: Souza TV, Malmonge SM, Santos AR. Bioprinting and stem cells: the new frontier of tissue engineering and regenerative medicine. J Stem Cell Res Ther. 2018;4(3):48-50. DOI: 10.15406/jsrt.2018.04.00114
Stem cells(SC) have great capacity for proliferation and differentiation and have been studied in different therapeutic approaches in cell therapy. However, the cells cannot organize themselves a three-dimensional environment similar to that found in tissues. In this context, tissue engineering appears which allows the cells to be organized three-dimensionally by scaffolds. Traditionally, scaffolds are produced by different methods and consist of dense, porous or fibrous structures. However, it was difficult until relatively recently to produce a material with characteristics and dimensions quite close to the injured tissue. Bioprinting is a technique that has been applied in tissue engineering and allows the solution of many of these limitations. Hydrogels based on natural polymers are known due to their favorable biocompatible properties, besides being biomaterials attractive for the cellular encapsulation. They provide an aqueous three-dimensional environment with biologically relevant chemical and physical signals mimicking the natural environment of the extracellular matrix. In our view, bioprinting is the latest frontier in tissue engineering as it enables the development of structures that can be used as implants as well as allowing tissue fragments and even more complex organs to be printed.
Keywords: bioprinting, biomaterials, tissue engineering, stem cells
Stem cells (SC) have great capacity for proliferation and differentiation. Some of their characteristics are self-renewal, the ability to differentiate into more than one cell line and the ability to originate functional cells in tissues derived from the same lineage.1 These important properties led several research groups to seek the use of stem cells as the main agent in the treatment of diseases, which has come to be called cell therapy. Promising results from various study groups have stimulated different centers around the world to initiate clinical trials that have investigated the feasibility of SC transplantation for the treatment of various diseases. Many interesting and other disappointing results have been reported.2
An important issue is that cells cannot in vitro organize themselves a three-dimensional environment similar to that found in tissues. In this way tissue engineering arises, which can be understood as the application of the principles of exact sciences and engineering for the creation and repair of tissues.3 There are three main elements in tissue engineering: 1) a scaffold, which is responsible for providing structural support for cell adhesion and growth, 2) the cells, in this case the stem cells have its importance recognized due to its differentiation potential, 3) the microenvironment, which in vitro ends up being represented by the culture conditions and factors used to induce or maintain cell differentiation.4
Usually scaffolds are produced by different methods and are composed of dense, porous or fibrous structures. Thinking of an implant situation, the porous and fibrous structures are of great importance because they allow the growth of the tissues and blood vessels, facilitating the tissue repair. However, it was difficult until not so long ago to produce a material with characteristics and dimensions similar to the injured tissue. This brought several limitations to the deployment of tissue-engineered products. These problems began to be solved with bioprinting.
Additive Manufacturing, better known as 3D printing, is a process of manufacturing three-dimensional objects from the digitally controlled deposition of successive layers of a given material in order to form a structure.5 On the other hand, Bioprinting aims the integration of living cells with biomaterials forming "living" three-dimensional structures, which allows the automated and reproducible production of functional three-dimensional living tissue, depositing layer-by-layer biocompatible materials with a cellular positioning of high precision6 as seeing in the Figure 1. That is, building the scaffold while living cells are deposited simultaneously. Bioprinting is a revolutionary strategy in the field of biomedical research since it allows the rapid and repeated fabrication of multifaceted tissues, being applied, among other purposes, to the creation of cartilage, skin, muscles and bones.7 This technology allows the controlled deposition of biomaterials that maintain the cellular viability, or the bioinks, in a three-dimensional plane, which allows the construction of complex scaffolds with distinct composition matrices, bioactive factors and cells that can acquire the native structure of the tissue where it will be implanted. In recent years, three-dimensional bioprinting has begun to become more accessible to researchers, allowing the technique to become widely used in tissue manufacturing.7,8 Three-dimensional parts can be bioprinted very precisely, which is critical when there is a need for reconstruction of specific parts such as nasal or auricular cartilage, in addition to dispensing with invasive procedures, such as the removal of cartilage from the ribs.9
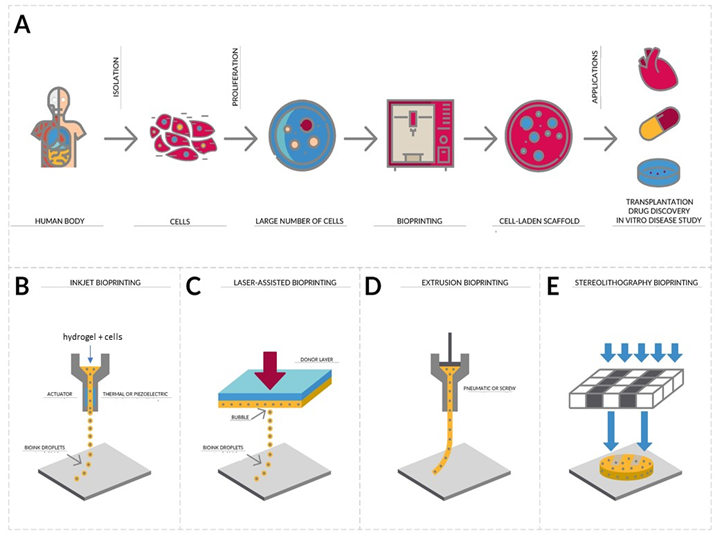
Figure 1 (A) Isolation, proliferation and application. Cells isolated from biopsy of a patient are used to form a bioink which is loaded into cartridges with a long extrusion nozzle for bioprinting.
A software drives the bioprinter to deposit a pattern of cell aggregates in precise layers. The printed is left to grow and mature and the hydrogel is removed. The printed tissue can be used in many different applications such as medical research, transplantation and drug discovery. There are different bioprinting strategies. (B) Inkjet bioprinting is a noncontact printing process that deposits precise picoliter droplets of bioink onto a hydrogel substrate or culture dish under computer control. (C) Laser-assisted bioprinting uses a laser as the energy source to deposit biomaterials onto a substrate. (D) The biomaterials are extruded by coordinating the motion of pneumatic pressure or plunger- or screw-based pressure in the form of a continuous filament through a microscale nozzle orifice or a microneedle onto a stationary substrate. (E) Stereolithography is a solid freeform, nozzle-free technology. A liquid, photo-sensitive polymer formulation is solidified upon illumination.13
As previously stated, Bioinks are formulations that allow the printing of living cells. The ideal formulation for bioinks must meet certain material and biological requirements. The properties of the materials are the printing capacity, mechanical structural strength, degradation and functionality. Biological requirements mainly include biocompatibility, cytocompatibility and bioactivity.10 Viscosity is a crucial parameter for bioink formulation as it affects print fidelity and cell encapsulation efficiency. High-viscosity polymer solutions are less likely to flow easily so the printed structure can maintain its shape for longer periods of time after printing.10 The choice of bioink is a factor of great importance when it comes to bioprinting. The scaffold material makes up a large part of the bioink formulation and this ends up affecting the ability of bioprinting, the growth and development of cells.11 Bioinks provide tissue-specific biochemical and physical stimuli to guide cellular behavior, such as migration, proliferation, differentiation, and maturation.12
Hydrogels are promising candidates for the development of bioinks because they are able to form networks of hydrophilic polymers, are biocompatible, have low cytotoxicity and their high water content allows them to acquire a structural similarity to the extracellular matrix.6,11 The most common hydrogels used in 3D bioprinting contain collagen, hyaluronic acid, chitosan and alginate. A hydrogel suitable for bioprinting must have sufficient viscosity to maintain the shape during bioprinting and be able to crosslink so that the structure is maintained after bioprinting. Crosslinking can happen by temperature change, can be ionic, physical (when there are non-covalent interactions, such as hydrophobic and electrostatic interactions or hydrogen bonds), chemical (requires the use of chemical crosslinking agents to form scaffolds through covalent bonds between chemical groups on the material) or UV light curing.6,11,13
In our view, bioprinting is the latest frontier in tissue engineering and regenerative medicine, as it enables the development of structures that can be used as implants, in addition to allowing fragments of tissues and even organs to be printed. Cells with great differentiation potential, such as stem cells, associated with well-developed bioinks may be the answer to the limitations we have to the reconstruction of tissues and organs that we currently have.
The authors would like to thank UFABC for TVS’s scholarship.
Author declares that there is no conflict of interest.

©2018 Souza, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.