Journal of
eISSN: 2475-5540


Research Article Volume 1 Issue 1
1Osteoarticular and Dental Regenerative Nanomedicine, Faculte de Medecine, France
2Universite de Strasbourg, Facult
Correspondence: Nadia Benkirane-Jessel, INSERM UMR1109, Osteoarticular and Dental Regenerative Nanomedicine, Faculte de Medecine, FMTS, F-67085 Strasbourg, France, Universite de Strasbourg, Faculte de Chirurgie Dentaire, 1 place de l?Hopital, F-67000 Strasbourg, France, Tel 33 3688 5337 6
Received: April 24, 2015 | Published: July 23, 2015
Citation: Keller L, Wagner Q, Pugliano M, et al. Bi-layered nano active implant with hybrid stem cell microtissues for tuned cartilage hypertrophy. J Stem Cell Res Ther. 2015;1(1):12-20. DOI: 10.15406/jsrt.2015.01.00004
Repair of articular cartilage defects remains challenging, as a majority of these defects reach the subchondral bone. The objectives of this work were first, to develop a new advanced therapeutic implant for osteoarticular repair, and second, to prevent hypertrophy of the articular cartilage region. For this aim, we developed a bi-compartmented implant, presenting a first layer for subchondral bone regeneration: Compartment 1, composed of a nanofibrouspoly (e-caprolactone) membrane equipped with BMP-7 growth factor nanoreservoirs, second layer for cartilage regeneration: Compartment 2, composed of alginate/hyaluronic acid hydrogel. To modulate hypertrophy, compartment 2 was seeded with microtissues of human mesenchymal stem cells co-cultured with human chondrocytes as differentiation inductors. This new strategy offers an appropriate double 3D environment (microtissues in Hydrogel) as inductive trigger for cartilage regeneration with tuned hypertrophy.
Keywords: regenerative nanomedicine, nanofibrous pcl implant, human mesenchymal stem cells (hmscs); microtissues (mts), hypertrophy, bmp-7
BMP, bone morphogenic protein; Bsp-II, bone sialoprotein-ii, CHI, chitosan; ECM, extracellular matrix; F, functionalized; FDA, food and drug administration; GAGs, glycosaminoglycans; HA, hyaluronic acid; hCHs, human chondrocytes; hMSCS, human mesenchymal stem cells; LbL, layer by layer; MTs, microtissues; NF, no functionalized; Ocn, osteocalcin; PEG, polyethylene glycol; PGA, poly glycolic acid; PLA, poly lactic acid; PCL, poly(e-caprolactone); Runx2, runt-related transcription factor 2; TGF, transforming growth factor
The articular cartilage is a very specific conjunctive tissue divided in several layers, from the articular zone on the articular surface to the mineralized subchondral bone in contact with the surrounding bone.1–3 To resist to the compression and provide a low-friction surface for the joint, the osteochondral unit presents a high specific extracellular matrix (ECM) organization in each layer, from high density GAGs (glycosaminoglycanes) absorbing water in the articular zone to a more collagen type I matrix in the subchondral bone.1–3 Due to the lack of vascularisation and cells, articular cartilage has a weak capacity for self-repair. Therefore, repair of osteochondral lesions still remains challenging, as the articular cartilage lesion also affects the subchondral bone underneath, and often results in fibrous and frangible repaired tissue.4–8 In order to increase the efficiency of articular cartilage repair, it is essential to first repair a solid subchondral bone, supporting articular cartilage regeneration on its surface.
Nanomaterials have a great potential in the field of regenerative medicine, especially in osteoarticular applications.1,9 For bone and cartilage, lots of materials have been developed to mimic the physiological extracellular matrix of tissues. They originate from natural substrates (collagen, alginate, gelatin, agarose, fibrin) or are synthetic materials (PEG (polyethylene glycol), PLA (poly lactic acid), PGA (poly glycolic acid).10,11 Recently, focus has been made on new generations of nanotechnology-based biomaterials.
As guidance offered by such materials allows the restoration of tissues by mimicking the ECM, nanofibrous scaffolds play a central role in modern strategies in nanomedicine.12,13 Our group has developed biodegradable electrospunnanofibrous materials using some FDA (Food and Drug Administration) approved polymer such as poly (e-caprolactone) (PCL).14,15 Furthermore, the functionalization of these new generation biomaterials, by incorporation of bioactive molecules, increases their efficiency to induce cell differentiation and tissue repair.14–32 To this end, we have recently developed new strategies for active therapeutic implants associating FDA approved components.14,15,33–36 We have reported the incorporation of active growth factors (TGFb, BMP-2, BMP-7) as a coating for nanofibrous scaffolds (synthetic or natural). The mechanism of the nanoreservoirs release of active therapeutics is cell contact dependent. These systems are already validated both in vitro and in vivo for the increase of the speed of bone regeneration.14,15,33–37
In recent years, in the field of bone and osteoarticular regenerative medicine, the use of adult mesenchymal stem cells (MSCs) provides an increasing interest. Derived from adult tissues, MSCs are defined as self-renewable, multipotent and immunosuppressive. Able to differentiate in chondrocytes or osteoblasts under specific conditions,38–41 these stem cells represent ideal candidates for the osteochondral regeneration. Indeed, with autologous chondrocytes, the procurement of a sufficient number of autologous cells is limited and these cells easily lose their phenotype in vitro.8,42,43 In recent years, it has been shown that MSCs cultured in a 3D structure, named “micromass” or “pellet”, have a more important capacity to differentiate in the chondrogenic way, compared to their use as single cells.44,45 Moreover, the use of spheroids (self-assembling microtissues) is known to mimic the embryonic condensation occurring during the embryonic endochondral development.46–49 Indeed, in recent studies, we have shown that hMSCs, used as single cells but also in 3D well-organized microtissues (MTs), were able to produce bone in vitro and in vivo when associated with a nanofibrous membrane equipped with BMP-7 nanoreservoirs.35,36
In the clinic today, repair strategies (microfracture, mosaicplasty etc…) mostly lead to the formation of fibrocartilage and have limited success, focusing on long-term repair limited to small lesions, and can reveal subchondral bone abnormality.4–8 To overcome these clinical drawbacks, osteochondral tissue engineering approaches using multi-layered materials for the bone-cartilage unit regeneration (subchondral bone together with articular cartilage) have recently emerged.7,50–55 In this work, we focused on subchondral bone and cartilage regeneration and developed an implant composed of two compartments: one for subchondral bone, and the other for articular cartilage regeneration.
To mimic the physiological cues of the bone-cartilage unit, we have used
Bioactive PCL nanofibrous membrane design
PCL nanofibrous membranes (50mm thick) were obtained by the electrospinning method as described previously.14,15 Addition of BMP-7 nanoreservoirs (CHI/BMP-7)6 on the nanofibers was performed by the Layer-by-Layer (LbL) technology.37 For that, the PCL membrane was dipped successively (6 times during 15min) in alternate solutions of Chitosan (CHI) (Protasan UP CL 113; Novamatrix, Sandvika, Norway; 500mg.mL-1) and BMP-7 (Euromedex, Souffelweyersheim, France; 200ng.mL-1) prepared in MES buffer (2-N-morpholino ethane sulfonic acid; Sigma-Aldrich Co., St Louis, MO, USA; 0.04M, NaCl 0.15M) at pH 5.5. Each bath was followed by a rinsing step in a solution of MES buffer (0.04M, NaCl 0.15M).
Cell culture
Human mesenchymal stem cells (hMSCs) and human chondrocytes (hCHs) (PromoCell, Heidelberg, Germany) were cultured in a proliferation medium (Promocell, Heidelberg, Germany) complemented with supplement mix serum (Promocell, Heidelberg, Germany), 50U.mL-1 of penicillin and 50µg.mL-1 of streptomycin. The cells were incubated at 37°C in a humidified atmosphere of 5% CO2. When cells reached sub-confluence, they were harvested with trypsin and sub-cultured to form microtissues (MTs). To form hMSCs MTs, 3 000hMSCs were cultured in each droplet in suspension of 40µL of medium in 3D culture plate system (GravityPLUS™ 3D Culture, InSphero AG, Zurich, Switzerland) during 5 days. To form hybrid MTs, 1000hCHs and 2000hMSCs were cultured in a droplet. The same number of formed MTs was then seeded in each nanofibrous PCL membrane (24MTs.cm-2) or in alginate/HA hydrogel.
Culture in alginate/HA hydrogel
Microtissues (MTs) were suspended in a solution of alginate (Sigma Aldrich) (12mg.mL-1) and hyaluronic acid (HA) (Lifecore Biomedical, Chaska, USA) (3mg.mL-1). The alginate/HA solution was then polymerized with a solution of CaCl2 (Sigma Aldrich) at 102mM, during 15min at 37°C. The microtissues embedded in alginate/ HA were then cultured in medium complemented with 1mM of CaCl2.
Microtissues differentiation
After seeding on PCL nanofibrous membrane functionalized (F) or not (NF) with BMP-7 nanoreservoirs, hMSCs MTs were cultured during 28 days in osteogenic medium consisting in Alpha-MEM complemented with L-glutamine (2mM), SVF (10%), fungizone (250U.mL-1), penicilline-streptomycine (10µ.mL-1), ascorbic acid (60mm), b-glycerophosphate (10mM) and dexamethasone (10nM). After seeding in alginate/HA hydrogel, hybrid MTs were cultured during 28 days in hMSCs proliferation medium (Promocell, Heidelberg, Germany), and hMSCs MTs were cultured during 28 days in chondrogenic medium consisting in Alpha-MEM complemented with L-glutamine (2mM), SVF (10%), fungizone (250U.mL-1), penicilline-streptomycine (10m.mL-1), L-Proline (40mg. mL-1), dexamethasone (10nM) and ascorbic acid (50µg.mL-1).
Indirect immunofluorescence
Samples were fixed with 4% paraformaldehyde (PFA) and were then treated with a solution of 0.1% Triton and 1% BSA for saturation and permeabilisation. Primary antibodies were then added during 2 hours at room temperature at the 1/200 concentration. Primary antibodies used were: rabbit anti-Runx2 (Sigma Aldrich), mouse anti-Osteocalcin (Ocn) (Santa Cruz Biotechnology, Dallas, TX, USA), and rabbit anti-BspII Sigma Aldrich). After rinses with PBS, 488 Alexa Fluor anti-rabbit or anti-mouse (Molecular Probes; Life Technologies, Fisher Scientific, Illkirch, France) (1/200) were added during 1 hour at room temperature. PBS rinses were made and phalloidin (Molecular Probes; Life Technologies) (1/200) was added during 20min at room temperature. A DAPI (Sigma-Aldrich) solution (200nM) was then added to the sample to observe cell nuclei. After mounting with Dako® (Dako, Courtaboeuf, France), the samples were observed with an epifluorescent microscope (Leica DM 4000B).
Alamar Blue® test
To analyse the metabolic activity of hMSCs MTs, the Alamar Blue® test (Thermo Fisher Scientific, Waltham, MA, USA) was used. For that, the cells were cultivated during 4 hours in a solution of 10% (v/v) Alamar Blue® in a complete medium without phenol red. Duplicates of each culture medium well were then analysed with a spectrometer (FC Multiskan) at the 570 and 595nm wavelengths. For each test, n=3. The p value was determined by a t-paired test.
Quantitative RT-PCR
For the MTs grown in the alginate/HA-polymerized hydrogel, samples were first dissolved in a solution of citrate (55mM), NaCl (0,15M) and EDTA (30mM). The total RNA was extracted from the MTs with the high pure RNA Isolation Kit (Roche). The RetroTRanscription was performed with the iScriptTM reverse Transcription Supermix (Bio-Rad, Marnes-la-Coquette, France). The Real-time PCR reaction were then carried out using the iTaqTM Universal SYBR® green super mix (Bio-Rad) and the CFX cycler system (Bio-Rad) with the following cycle condition: an initial denaturation step of 95°C for 2 min was performed, followed by 39 cycles of denaturation at 95°C for 5 seconds, annealing at 60°C during 30 seconds and extension at 65°C for 5 seconds. For each test, n=3. Statistical significance was determined by a t paired test (Table 1).
Target gene |
Forward |
Reverse |
ACTIN B |
5’-GATGAGATTGGCATGGCTTT-3’ |
5’-CACCTTCACCGTTCCAGTTT-3’ |
RUNX2 |
5’-CCAACCCACGAATGCACTATC-3’ |
5‘-TAGTGAGTGGTGGCGGACATAC-3’ |
Table 1 The primers used
In vivo experimentation
Animals and tissues: All procedures were designed in compliance with the recommendations of the European Union (2010/63/EU) for the care and use of laboratory animals. Ethics statement: Experiments followed current European Union regulations (Directive 2010/63/EU), and were performed according to authorized investigator Dr. N. Jessel (Director of the “Osteoarticular and Dental Regenerative Nanomedicine” Team), holder of a personal license from “Prefecture du Bas-Rhin” (No. 67-315), who oversaw experiments done on mice. All experiments were done in the “Animalerie Centrale de la Faculte de Medecine de Strasbourg” with the approval number: A 67-482-35 from the Veterinary Public Health Service of the “Prefecture du Bas-Rhin”, representing the French Ministry of Agriculture, Department of Veterinary Science. For further tissues implantations, all surgery was performed under Ketamine and Xylazine anesthesia, and all efforts were made to minimize suffering. Nude male mice (CTR: NIH-Foxn1nu, Charles River, France), 6 weeks of age, were anesthetized with intra-peritoneal injection of 100mg.kg-1 of ketamine (VIRBAC Sante Animale, Centravet) and 10mg.kg-1 of Xylazine (Rompun® 2%, Centravet). The nano structured bioactive implant was implanted behind the ears of mice, between skin and muscles. After 90days, the animals were sacrificed with an intra-peritoneal injection of lethal dose of ketamine, to examine the explant by histological staining.
Histological staining of glycosaminoglycanes
Samples of alginate hydrogel were first fixed with a solution of 4% para formaldehyde (PFA) containing 100mM of cacodylatetrihydrate and 10mM of CaCl2. Samples were then embedded in paraffin for serial sections of 7mm. For Safranin O/ Fast green staining, the samples were rinsed with PBS and dipped in a solution of Fast green (0,02% m/v), acetic acid (1% m/v) and finally Safranin O (0,1% m/v). For Alcian Blue staining, the samples were rinsed with distilled water and a solution of 2% (m/v) Alcian Blue (Sigma-Aldrich) at pH 4.2 was added during 2 hours at room temperature. Samples were then rinsed with distilled water, dehydrated and mounted with Histolaque LMR® (Labo Moderne, France) to be observed with bright field microscope (Leica DM 4000B).
PCL membrane equipped with BMP-7 nanoreservoirs for subchondral bone repair (compartment 1)
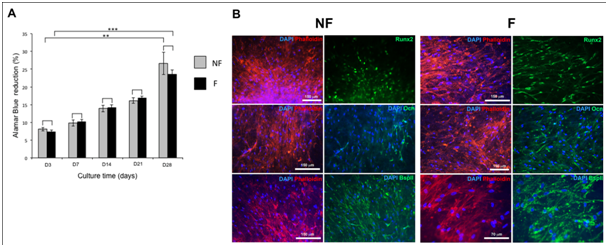
In bone regenerative medicine field, bioactive biomaterials mimicking the extracellular matrix have been developed.56,57 As examples, Medtronic offers InductOs®, which is a basic collagen matrix (of animal origin) soaked in osteogenic growth factor BMP-2 (12mg), and Stryker® offers the same collagen membrane soaked in BMP-7.58,59 Despite their application in clinic, the release of these active molecules from these materials is passive and not controlled. Recently, we have developed a nano reservoirs technology, on synthetic or natural membranes14,15,33,35,36 with active cell contact dependent release. The advantage of this technology resides in the fact that this material generates an active release of molecules dependent on the cells’ adhesion on the nano reservoirs and their cell-dependent degradation,14 leading to an increase of growth factor bioavailability after implantation. For bone tissue repair, we have previously shown that BMP-7 nano reservoirs on collagen membrane (Bio Gide® from Geistlich) were able to increase mineralization of human osteoblasts and mesenchymal stem cells (hMSCs), seeded as single cells or as microtissues (MTs).35,36 The replacement of this natural material (of animal origin) by a synthetic polymer, can be a key to avoid inflammatory response and rejection in vivo. For that purpose, we developed electro spunnano fibrous membranes based on the poly (e-caprolactone) (PCL), which is an FDA approved polymer.14,15 This material, when equipped with nano reservoirs of BMP-2, can increase the speed of bone marker expression in vitro after 14 days and in vivo.14,15 As a strategy to enhance subchondral bone regeneration efficiency in vivo, we used BMP-7 functionalized (F) nanofibrous PCL membrane and human mesenchymal stem cells. To confirm the osteogenic differentiation of hMSCs MTs on functionalized (F) PCL membrane, these hMSCs MTs were cultured in osteogenic medium during 28 days (Figure 1). The PCL (F) membrane was biocompatible with hMSc MTs, as the cells metabolic activity increased all along the culture (Figure 1A). In the literature, it is reported that BMP-7 acts as an attenuator of cell cycling during early osteogenic differentiation of hMSCs60 and our results are in accordance with previous observations.35,36 After 14 days, hss MTs were able to express bone specific markers (Runx2, Ocn, BspII) on the (NF) and (F) PCL membrane (Figure 1B). Runx2, a transcription factor induced when mesenchymal cells are in the bone differentiation way was expressed in the two conditions (F, NF). Interestingly, Runx2 and Osteocalcin (Ocn) seem to be more important in the presence of the nanoreservoirs (NRs) (Figure 1B). These results were in accordance with expected results as we previously showed the osteogenic differentiation of hMSCs as single cells with BMP-7 NRs,36 or as MTs.35 As collagen represented a good tool for hMSCs MTs differentiation,35 the PCL electro spunnano fibrous membrane equipped with BMP-7 nano reservoirs can represent a good support for these cells and for bone regeneration in vivo. With this strategy, we can overcome the current approaches used in the clinic today (Stryker collagen soaked in BMP-7) for bone repair, leading to overdosing due to the massive and passive release of BMP-7, inducing adverse side effects.
Hybrid MTs in alginate/HA hydrogel for cartilage regeneration with tuned hypertrophy (Compartment 2)
To engineer cartilage on the bone surface functionalized support (compartment 1), we used Alginate/HA hydrogel seeded with stem cells to mimic the articular cartilage compartment. First, hMSCs in well-organized MTs were used to mimic the condensation during the endochondral differentiation way during embryonic development.46–49 Already used in the cartilage-engineering field, MSCs cultured in a 3D configuration (micromass, pellet) show increased chondrogenic differentiation compared to MSCs single cells.44,45 Moreover, it was shown that co-culture of MSCs with chondrocytes induces a chondrogenic differentiation of MSCs with less hypertrophic markers.61–64 On the other hand, single cells and 2D culture systems can dedifferentiate chondrocytes via fibroblastic phenotype.65,66 Rather than performing culture with growth factors, culture modes with cellular interactions have been shown to generate more physiological cues and doses. The paracrine signal between chondrocytes and MSCs is dependent on the distance between cells and an excessive distance blocks their interaction.67
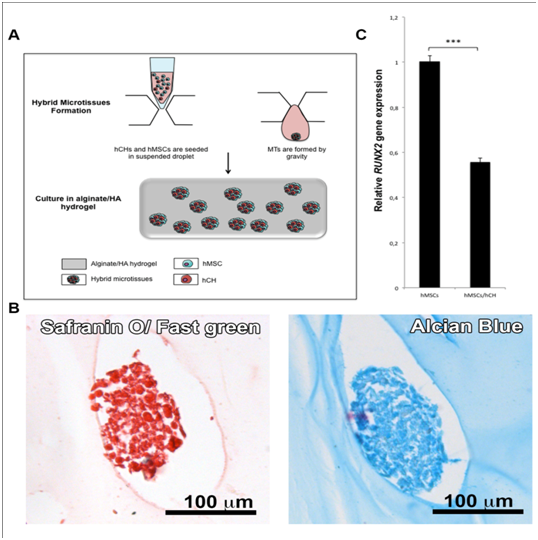
In this work we chose to increase these cell-cell interactions by using a co-culture of two kinds of cells (hMSCs and hCHs) in microtissues (MTs). Having shown that hMSCs MTs in HA/hydrogel can represent a good tool for chondrogenic differentiation, the next step was to analyse the effect of hCHs on hMSCs in MTs (Figure 2). For that purpose, hybrid MTs (hMSCs/hCHs) were cultured in alginate/HA hydrogel during 28 days in non chondrogenic medium (MSC proliferation medium). The glycosaminoglycanes (GAGs) expression and the hypertrophic marker Runx2 were then evaluated in the MTs (Figure 2B & 2C). We first observed the chondrogenic differentiation of these hybrid MTs by staining specific GAGs (Figure 2B). QRT-PCR was then used to compare the expression of RUNX2 in hybrid MTs and in hMSCs MTs, and revealed a significant decrease in RUNX2 in the MTs containing hCHs (hybrid MTs) (Figure 2C). The use of hCHs in combination with hMSCs in MTs allows inducing a less hypertrophic phenotype. Alginate polymeric material was already shown as beneficial for cartilage engineering and repair, and has been used for the transplantation of cells in several clinical trials.11,68,69 Indeed, this unmodified hydrogel, which has attractive properties such as immobilization of cells, can also benefit to the chondrogenic differentiation because it does not promote mammalian cell attachment and proliferation.70,71 Using alginate hydrogel to mimic the natural environment of articular cartilage, associated with co-cultured hMSCs/hCHs in a tridimensional well organized conformation (hybrid MTs), is a good strategy to regenerate articular cartilage tissue with less hypertrophy.
Nano functionalized compartmented implant design (compartment 1+ compartment 2)
A majority of articular cartilage defects reach the subchondral bone and articular cartilage repair is done on frangible subchondral bone.4–8 To increase the efficiency of cartilage repair we have used, not only an alginate hydrogel (compartment 2) seeded with hybrid MTs for cartilage repair, but also a functionalized membrane (compartment 1) for subchondral bone regeneration. By using this innovative strategy, our proposed therapeutic medical device associates two compartments in order to reproduce the bone-cartilage unit (Figure 3A).
For the design of the compartment 2, we have used alginate hydrogel seeded with hybrid MTs (hMSCs and Chondrocytes: hCHs). Autologous chondrocytes are used in conventional cell-based therapy, but these techniques are associated with several limitations such as the number of cells available and dedifferentiation in 2D culture.8,42,43 By associating hCHs and hMSCs in MTs without the need of chondrogenic medium, we were able to produce chondrogenic MTs, and to prevent their hypertrophy (Figure 2). We assume that by associating hybrid MTs, alginate/HA hydrogel and a membrane equipped with BMP-7 nanoreservoirs, as a new advanced therapeutic medicinal device, we could regenerate first a robust subchondral bone, itself sustaining stable articular cartilage regeneration on its surface.
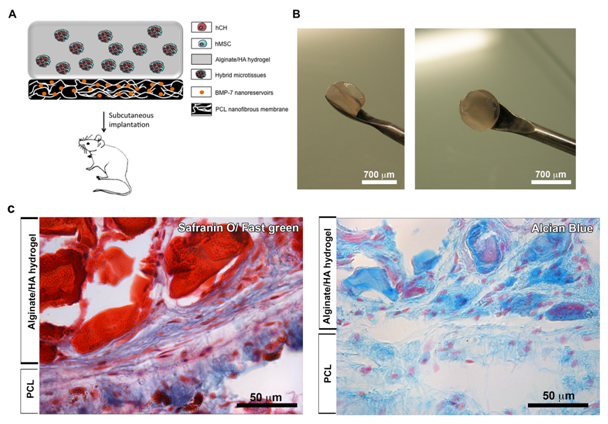
To demonstrate the behavior of these hybrid MTs in vivo on the membrane (F), we subcutaneously implanted our nanostructured therapeutic medical device (Figure 3A & 3B). After 90 days, implants were retrieved and cartilage specific staining (GAGs) of the samples was performed (Figure 3C). The two compartments of the implant were visible after 90 days in vivo, showing the bone part of the implant in green (Safranin O/ Fast green) (compartment 1) and the GAGs functional compartment visible in blue (Alcian Blue) or in red (Safranin O/ Fast green) (compartment 2) (Figure 3C). During implantation time, these two compartments were vascularized and cells coming from the microtissues migrated all along the alginate structure and membrane, showing viability of these associated structures (Figure 3C).
In this manuscript, we used not only a nano fibrous electrospun membrane fortified with active nano reservoirs of therapeutics (BMP-7) for subchondral bone repair (compartment 1) but also an appropriate 3D environment for mesenchymal stem cells based on an hydrogel and microtissues for cartilage regeneration with less hypertrophy. These hMSCs were co-cultured with human chondrocytes (hCHs) as inductors in microtissues and placed in some alginate/HA hydrogel (compartment 2). Based on RUNX2 expression, we show that with this double 3D strategy, we are able to modulate the hypertrophic phenotype of the differentiated cells. We have as well-shown in vivo subchondral bone and cartilage regeneration by combining both compartment 1 and 2.
Success of osteochondral defect treatment can be achieved by targeting not only cartilage repair but also robust subchondral bone. By associating human mesenchymal stem cells/bioactive nano structured material, with alginate hydrogel/3D well-organized co-cultured hMSCs, we are able to engineer an implant designed for the articular cartilage but also for the subchondral bone tissue repair. By using a nano fibrous bioactive PCL membrane, and alginate hydrogel combined with stem cells and chondrocytes microtissues, we enhance the cell-cell interactions and optimize the micro environment for subsequent cartilage-bone regeneration. Furthermore, with the use of hybrid microtissues (hCHs, hMSCs) in double 3D environment (alginate /HA, MT), we were able to modulate the hypertrophy of the cells in the articular cartilage compartment. These results demonstrate a promising approach to develop a nano structured and bioactive implantable medical device in the field osteoarticular regenerative nano medicine. Our proposed advanced therapeutic medicinal device opens a new strategy for cartilage repair combining the double 3D active environment of stem cells (Hybrid microtissues in hydrogel) and the nanofibrous active membrane for subchondral bone repair. Our innovative technology based on cells microtissues and hydrogels, as a double 3D environment, is an adaptable advanced therapeutic medical device. This technology can be tuned by using different kinds of cells, active drugs and polymers (natural or synthetic) and thereby applied for the regeneration of other tissues such as skin and vasculature.
This work was supported by the project Nano OSCAR from the “Agence Nationale de la Recherche”, ANR and SATT Conectus. We are indebted to “Faculté de Chirurgie Dentaire” Strasbourg for financial support.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.

©2015 Keller, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.