Journal of
eISSN: 2475-5540


Research Article Volume 4 Issue 1
1Bennu Life Regenerative Institute, USA
2Department of R&D and Celling Biosciences, USA
Correspondence: Meghana Malur, Celling Biosciences, 4719 South Congress Avenue, Austin, Texas, USA, Tel 5129682553, Fax 5126372096
Received: October 16, 2017 | Published: February 7, 2018
Citation: Pettine KA, Suzuki RK, Murphy MB, et al. Autologous bone marrow concentrate (bmc) for the treatment of osteoarthritis (o.a) of the knee, hip, and shoulder in former n.f.l. players. J Stem Cell Res Ther. 2018;4(1):9-13. DOI: 10.15406/jsrt.2018.04.00106
The use of bone marrow concentrate (BMC), to treat Osteoarthritis (OA) is the standard of care in veterinary medicine. There remains a paucity of clinical data on the safety and efficacy of utilizing BMC in humans. Fifty-one former N.F.L. players were injected with BMC for shoulder (n=30), hip (n=11) or knee (n=10) osteoarthritis. All were surgical candidates. At one year follow-up up the average shoulder patient improved 68% in VAS, 64% in Quick DASH and 40% in ASES. The average hip patient improved 70% in VAS, 72% in ODI and 64% in Lower Extremity Functional Scale (LEFS). The average knee patient improved 70% in VAS, 67% in ODI and 62% in LEFS. All improvements were to a p<0.001. There were no complications and no patient was made worse. Only (1/30) shoulder, (1/11) hip and (2/10) knee patients elected to have surgery at minimum 1year follow-up.
Keywords: autologous, regenerative medicine, osteoarthritis, bone marrow concentrate, mesenchymal stem cells, national football league
BMC, bone marrow concentrates; N.F.L, national football league; O.A, osteoarthritis; ODI, oswestry disability index; VAS, visual analog scale; LEFS, lower extremity functional scale; QD, quick dash; ASES, american shoulder and elbow surgeons
Osteoarthritis (O.A) affects over 50million Americans. This includes mostly O.A of the shoulder, hip, and knee. Shoulder osteoarthritis (O.A) has been demonstrated in cadaver and radiographic studies to affect up to 33% of patients over the age of 60.1 Patients with shoulder O.A have pain, crepitus, decreased ability to place their hand at a desired point in space and loss of motion, severely impairing activities of daily living. The nonsurgical treatments for shoulder O.A include the use of analgesics, non-steroidal anti-inflammatory medications, and shoulder exercises to maintain range of motion. The surgical treatment for shoulder O.A is total shoulder arthroplasty.1,2
The American Academy of Orthopedic Surgeons (AAOS) recommended treatment for O.A of the hip and the knee includes the following: weight loss, gentle exercise, and anti-inflammatory medications followed by total hip or knee replacement. The AAOS does not recommend arthroscopic debridement or any Hyaluronic acid products such as Synvisc®, Euflexxa™, Orthovisc®, Supartz™, or Hyalgan® for treating hip and knee osteoarthritis. Four prospective randomized studies have shown no benefit over placebo at six-month follow-up with these Hyaluronic injections. Despite the fact Hyaluronic acid products have shown no efficacy, the market for these products is several hundred million dollars per year.3–8 The reason for this is the huge void between non-operative treatments and the only surgical treatment, total hip, or knee arthroplasty. Last year in the United States, over 1million total hip and knee replacements were performed with a direct cost of over $30billion.9,10 These numbers are expected to double in the next three years (AAOS.org).9 Every day, 10,000 people in the United States turn 65 and this will continue for the next 14years (aarp.org) increasing the population that will be in the need of total joint replacements.
Treating O.A with BMC is standard of care in veterinary medicine, primarily in dogs and horses.11–14 Research has shown that Mesenchymal Stem Cells (MSCs) obtained from BMC are superior to MSCs obtained from adipose.15 There are animal studies and clinical data documenting the efficacy of injecting BMC into an arthritic joint.16–20 The primary problem in orthopedics is a lack of blood supply to the articular cartilage of the joint.1,2 Thus, the ability of the cartilage cells to repair damage is very limited. MSCs obtained from BMC have many positive attributes. MSCs are anti-inflammatory, secrete numerous growth factors, stimulate blood vessel formation, and modulate the body’s immune system to enhance healing.21 Delivering MSCs directly into the affected joint may provide therapeutic benefit.
The primary objective of this study was to determine the safety and efficacy of performing an intra-articular injection of BMC to treat shoulder, hip, and knee O.A.
Study design
This study is a prospective, open label, non-randomized evaluation of autologous single BMC injection for the treatment of O.A of the shoulder, hip, or knee. All patients were counseled and consented as per IRB requirements. The treatment was offered free to study participants. The study protocol for the hip and shoulder was approved by an Institutional Review Board (IRB). Fifty-one patients were enrolled in this study. The study was performed at a single center. The details of the study design, patient demographics, bone marrow collection and processing, injection procedure and statistical tests performed are described.
All patients were former National Football League (N.F.L.) players with a primary complaint consistent with moderate to severe shoulder, hip, or knee O.A. The patients were required to have at least grade-2 changes utilizing the Kellgren-Lawrence scale for O.A.22 Patients underwent a pre-injection medical history and physical examination of their shoulder (shoulder functional scale and Visual Analog Scale(VAS)) or their hip or knee (lower extremity functional scale (LEFS), Oswestry Disability Index (ODI) and VAS). In patients with bilateral O.A, only the most symptomatic shoulder, hip, or knee was injected. Follow up was obtained at three months, six months and 12 months following the procedure.
Shoulder O.A was defined by pain and stiffness in the shoulder joint worsened by exercise and decreased shoulder range of motion. O.A in the hip or knee was defined by pain and stiffness, worsened by exercise and weight bearing. Patients being treated for shoulder O.A had radiographs of the shoulder to rate them as Kellgren -0,-1,-2,-3, or -4 on the Kellgren-Lawrence scale. There were seven patients with grade 4, 17 patients with grade 3 and 6 patients with grade 2 O.A. All patients being treated for hip and knee O.A, had standing AP and lateral radiographs as well as MRI scanning to rate them as 0, 1, 2, 3, or 4 on the Kellgren-Lawrence Scale. For the hip, there were 9 patients with Kellgren-Lawrence grade 3 changes, 1 patient with grade 2 changes, and 1 with changes in between grade 1 and 2. For the knee, there were 8 patients with Kellgren-Lawrence grade 3 changes, and 2 patients with grade 2 changes. Grade 2 changes are moderate, and grade 3 changes are moderate to severe, Grade 4 is bone on bone.
Bone Marrow Collection and processing
Bone marrow aspirate (BMA, 55 mL) was collected over acid citrate dextrose-anticoagulant (ACD-A, 5ml) from the patient’s posterior iliac crest. The procedure was performed with IV sedation consisting of Versed and Fentanyl. Positioning of the Jamshidi needle in the iliac wing was confirmed by fluoroscopy. BMA was collected in a 60mL syringe in a series of discrete pulls on the plunger (targeting a collection of 5-10mL per full), with repositioning of the needle tip between pulls based on the reported enrichment of progenitor cells by Hernigou.23 The BMA was processed using the ART21 system (Celling Biosciences, Austin, TX) to produce a bone marrow concentrate (BMC) cell preparation. The 55mL of BMA was centrifuged for 12minutes to produce 7mL of BMC. The 7mL of BMC was drawn from the processing device, and glucose and bicarbonate were added to the 7mL of BMC. The appropriate volume of BMC with the additives was immediately transferred to the physician for injection into the shoulder, hip or knee of the enrolled patient.24 Processing disposables were provided at no cost by Selling Biosciences (Austin, Texas, USA) without any further financial contributions to the study or principal investigator.
Patient demographics
The number of patients undergoing treatment for shoulder, hip, and knee along with average BMI and average age are shown in Table 1. All patients were candidates for total replacement of shoulder, hip, or knee.
Shoulder |
Hip |
Knee |
|
Number of patients |
30 |
11 |
10 |
Average BMI |
29.8 |
30.5 |
29.4 |
Average Age |
58.7 |
60.3 |
59.8 |
Table 1 Description of Patient Demographics.
Shoulder, hip, and knee injection
All shoulders (n=30), hips (n=11) and knees (n=10) were sterilized with betadine skin prep. Nineteen patients were injected in the right shoulder and eleven were injected in the left shoulder. Under fluoroscopic control a 20-gauge needle was placed through either an anterior or posterior approach into the glenohumeral joint. The needle was placed against the humoral head within the shoulder joint capsule. Needle placement was verified under fluoroscopic control. At this point 7mL of BMC was placed into the shoulder joint. The entire procedure of bone marrow harvesting, concentrating and shoulder, hip, or knee injection required 30 minutes on average.
Under fluoroscopic control, a 20-gauge needle was placed through an anterior approach into the hip joint or through an anterior peripatellar tendon approach into the knee compartment. Needle placement was verified with fluoroscopy. At this point, 5ml of bone marrow concentrate were placed into a single hip joint or into a single knee joint.
Patients were prescribed pain medications to use as needed for 3days. They were put on a restricted physical activity for 2weeks following the procedure. Passive low-resistance range of motion was encouraged immediately. After two weeks, patients were allowed to return to full activity.
Cell analysis
Cell analysis and characterization of all 51 BMC samples were performed. An aliquot (1ml) of each subjects’ BMC was packed in a shipping container with 5°C cold packs and shipped overnight to the cell analysis laboratory (Selling Biosciences, Austin, Texas). The samples were received and processed immediately to determine the total nucleated cell count and viability using a Nucleocounter NC-100 (Chemomtec, Denmark). The BMC was diluted in phosphate buffered saline (PBS, Invitrogen, Grand Island, NY) with 2% fetal bovine serum (FBS, Hyclone human mesenchymal grade, Thermo Scientific, Waltham, MA) and subjected to a Ficoll-Paque (GE Healthcare Life Sciences, Piscataway, NJ) gradient separation (1:1 cell solution to Ficoll ratio by volume) to deplete the red blood cells. Analysis of the recovered cells included performing colony forming unit-fibroblast and osteogenic (CFU-F and CFU-O, respectively) assays. The CFU-F assay was performed by creating a dilution series (in culture medium with 5% FBS and 1% antibiotics) of each cell preparation at concentrations of 50,000 to 500,000 total nucleated cells (TNC) per well in standard 12-well plates. The plates were placed in an incubator at 37°C, 5% CO2 and 100% humidity for 72hours when the medium was replaced. Medium was replaced every 3 days. After 9days in culture, wells were gently washed with PBS, the colonies/cells were fixed with methanol, the attached cells were stained with Crystal Violet, and cells were rinsed with water followed by air-drying the plates. Visualization and counting of the colonies was done with an inverted microscope. Colonies containing 20 or more cells were scored as a CFU-F. The CFU-O assay was performed identically as CFU-F, but after 9days the medium was changed to an osteogenic induction medium (Advance STEM Osteogenic Differentiation Kit, HyClone, Logan, UT) for an additional 9days with complete medium change every 3days. On day 18, the wells were washed with PBS, then fixed for 15minutes in 2% formalin solution and co-stained for alkaline Phosphatase activity (Vector Blue ALP, Vector Labs, Burlingame, CA) and calcified extracellular matrix (0.5% Alizarin Red solution, Sigma-Aldrich, St. Louis, MO).
Statistical tests
Univariable data comparisons were analyzed by two-tailed Student’s t-test with a 95% confidence interval (α=0.05. Microsoft Excel). Multivariable data were evaluated by analysis of variance (ANOVA) using JMP 9 statistical analysis software (SAS Institute, Cary NC).
Results
No patient was made clinically worse from the bone marrow harvesting or the BMC injection. There was no change in the radiographic appearance of the O.A joint in any patient. Through one year follow-up, the average patient experienced statistically improved outcome measures as shown in Figure 1. Approximately 50% of patients had increase in range of motion in the shoulder and decreased signs of tendon swelling (based on the physician’s exam section of the ASES shoulder score for up to one year post injection.). The three patients with severe rotator cuff arthropathy and concurrent Grade 4 O.A did not show significant clinical improvement, and one elected to have a total shoulder arthroplasty.

Figure 1 Patient progress versus time after BMC injection into the Shoulder: Average (Avg.) QD = quickDASH, a measure of shoulder function with lower scores being ideal. ASES = American Shoulder and Elbow Surgeons – Shoulder score, measures shoulder function also (and includes a physician assessed component), Higher percentages are better scores. VAS = Visual Analog Scale scores – Higher scores indicate higher pain, p<0.001
One year after BMC injection, the average improvement in hip pain was 83% (VAS) and function was 77.7% (LEFS) (Figure 2) and the average improvement in knee pain was 70.1% (VAS) and function was 63.8% (LEFS) (Figure 3). One patient proceeded with a total hip replacement and two patients elected to proceed with total knee arthroplasty at 1 year follow up.
The cells had good viability (shoulder=95%, hip=92.9% and knee=93.2%) and the CFU-F and CFU-O/ml counts and total nucleated counts (TNCs) are shown in Table 2.
Shoulder |
Hip |
Knee |
|
TNC |
9.86*107 |
5.59*107 |
5.47*107 |
Viability |
95% |
92.90% |
93.20% |
CFU-F (Counts/mL) |
3613 |
925 |
1148 |
CFU-O (Counts/mL) |
-* |
1435 (n=8*) |
1624 (n=9*) |
Table 2 Average (i) Total nucleated cell counts (TNC), (ii) Viability (iii) CFU-F/ml of Bone Marrow Concentrate, and (iv) CFU-O/ml of bone marrow concentrate (BMC) of former N.F.L. players being treated for shoulder (n=30), hip (n=10), or knee (n=10) Osteoarthritis
*Cell numbers on some samples were not in the appropriate range for CFU-O assays.
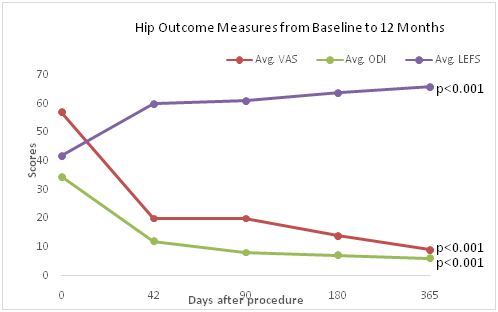
Figure 2 Patient progress versus time after BMC injection into the Hip: Average (Avg.).
VAS: Visual Analog Scale scores – Higher scores indicate higher pain; Avg. ODI: Oswestry Disability Index; Avg. LEFS: Lower Extremity Functional Scale, p<0.001.
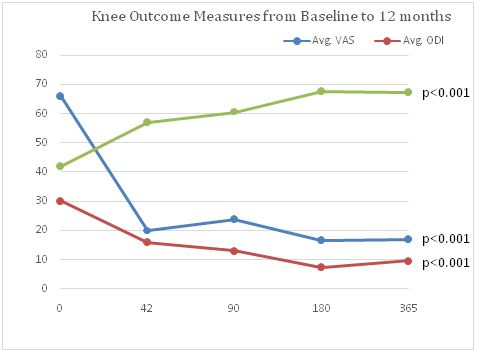
Figure 3 Patient progress versus time after BMC injection into the Knee: Average (Avg.).
VAS: Visual Analog Scale scores – Higher scores indicate higher pain; Average ODI: Oswestry Disability Index; Average LEFS: Lower Extremity Functional Scale, p<0.001.
Discussion
This preliminary study shows safety and efficacy in treating O.A of the hip, knee, and shoulder with a single injection of autologous BMC. One year post BMC injection, (i) only 1 of the 30 patients who had shoulder O.A, (ii) only 1 of the 11 patients who had hip O.A, and (iii) only 2 of the 10 patients who had knee O.A, underwent surgery. The regular use of analgesics and NSAIDs were decreased on average 60% after the single injection of BMC for up to one year in all the groups. The average shoulder patient improved 68% in VAS, 64% in Quick DASH and 40% in ASES. One patient with grade 4 rotator cuff arthropathy underwent surgery. The average hip patient improved 69.8% in VAS, 72.1% in ODI and 63.6% in LEFS. The average knee patient improved 70.1% in VAS, 67% in ODI and 62.1 % in LEFS.
Limitations of this study include the small number of patients and one year follow up. There were no improvements in the radiographic appearance of any of the treated joints despite clinical improvements. The study was not randomized although 22/30 patients had bilateral shoulder O.A (8/10 had bilateral knee O.A and 9/11 had bilateral hip O.A).
No patient showed improvement in the non-injected shoulder, hip or knee during the year follow-up which could be considered a control. There were no complications associated with the bone marrow harvesting or injection of the BMC.
The results of this study indicate it may be reasonable to consider an injection of autologous BMC into a shoulder, hip, or knee with O.A prior to a patient undergoing a total shoulder, hip, or knee arthroplasty.
Fifty-one former N.F.L. players underwent a single injection of bone marrow concentrate (BMC) into an arthritic shoulder (30 patients), hip (11 patients) or knee (10 patients) with one year follow-up. The average shoulder improved 68% in VAS, 64% in Quick DASH and 40% in ASES. The average hip improved 70% in VAS, 72% in ODI and 64% in LEFs. The average knee improved 70% in VAS, 67% in ODI and 62% in LEFS. One patient had a total shoulder arthroplasty(0.03%), one patient had Total Hip Arthroplasty (0.09%) and two patients had Total Knee Arthroplasty (0.2%). No patient was made worse. These results indicate a BMC injection into an arthritic joint is safe and efficacious and should be considered prior to joint replacement.
The authors would like to acknowledge the retired N.F.L. players association for their strong interest in pursuing stem cell therapy for treating their football-related injuries.
The authors have no conflict of interest.

©2018 Pettine, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.