Journal of
eISSN: 2475-5540


Research Article Volume 4 Issue 4
1Research and Development, CelluGen Biotech, India
2PG & Research Centre in Biotechnology, Periyar University, India
3PG & Research Centre in Microbiology, Periyar University, India
4Centre for Research & PG studies in Botany, Thiagarajar College, India
Correspondence: Jaianand Kannaiyan, Research and Development, Cellu Gen Biotech, MGR College, Periyar University, India, Tel +91 9910005112, Tel +91 9843907474
Received: September 27, 2018 | Published: October 18, 2018
Citation: Kannaiyan J, Veeramanikandan V, Muthukumarasamy E, et al. An in-vitro study of Amniotic membrane, villous chorion and Wharton’s jelly-derived Mesenchymal stem cells and their potential for cardiac repair. J Stem Cell Res Ther. 2018;4(4):104-110. DOI: 10.15406/jsrt.2018.04.00124
Background: Research in the past decade has improved our understanding of the regenerative capacity of MSC. Fetal MSC's which are known to be exhibiting immunosuppressive properties can be advantageous for allogeneic transplantation as well. Today across the globe there are many deadly diseases spreading rage over, myocardial disorders are one of them. Major loss of native functional cardiomyocytes which are able to regenerate damaged necrotic tissue to compensate for cardiac dysfunction; leads to myocardial disorders. Transplantation of cardiac progenitors which will ultimately replace damaged or lost cardiomyocytes as an intervention has shown promising development as a therapeutic modality. Besides it is proved that the paracrine release of bioactive molecules by MSC's contribute to improved heart function via pro-survival effects on host cardiomyocytes initiating an angiogenic response in the infracted myocardium.
Materials and methods: We tested whether MSCs isolated from three fetal tissue sources such as Amniotic membrane, Wharton's jelly, and Villous Chorion can be coaxed in-vitro into mesodermal and cardiomyogenic lineages and, angiogenesis with the exposure to 5’Azacytidine and, confirmed with the expression of cardiac transcription factor markers such as CSM1, Cardiac Troponin (cTnT) of these differentiated cells.
Results: Based on these studies, we have proved that all different fetal sources were capable of differentiating into cardiac progenitors MSC's and angiogenesis capacity.
Conclusion: Our data suggest MSC's pooled fetal source is a rich and non- controversial source, which can be differentiated into cardiac progenitors expressing cells.
Keywords: amniotic membrane (AM), mesenchymal stem cells/multipotent stromal cells (MSCs), villous chorion (VC), wharton’s jelly (WJ), Cardiac markers CSM1, cTnT
Myocardial disorders such as acute myocardial infarction endanger millions of people and cause a substantial number of deaths each year; 1 of every 6 deaths all over the world. After an acute myocardial infarction (MI), the heart has a limited capacity of self-renewal undergoing remodelling with resulting left ventricular dysfunction (LVD).1,2 Despite the improvement in several pharmacological, interventional and surgical therapeutic measures, the prognosis for heart failure patients remains very poor. Although an early wave of clinical trials has generated marginal success; medical researchers and regulators face new issues and uncertainties involving long-term safety and efficacy. Put together these observations; account for the continued search for new optional treatments. Among many alternatives, cellular therapy has gained a growing interest as the new field exploiting cells to generate biological substitute, improve tissue function and thereby restoring the damaged tissue with high proliferability and differentiability.3,4 It is also being focused as a potential alternative to complicated tissue or organ transplantation because several factors have been cited as causes for disparities in organ donation and its successful transplantation such as lack of education, religious beliefs, distrust of the health care system, lack of communication between healthcare providers and minority patients and desire of minority members to donate their organs only to members of their same ethnic classification.
Since the pioneering work of Friedenstein et al.5 regenerative properties of Mesenchymal stromal cells (MSCs) are well understood and progressed rapidly.5 Recent data has also suggested therapeutic benefits of MSC’s supplemented with other properties such as homing to the damaged tissue, regulating immune and inflammatory responses at the targeted sites and thereby facilitating repair of the degenerated tissue. These specificities are making them very unique and lucrative candidates for cell-based therapies obviating the market need for their large-scale isolation. Over the period of time during a search for an alternative cellular medicine; an abrupt growth has been observed for MSC's as a future cell-based therapeutic strategy for cardiac repair. Various pathways of MSC mediated cardiac improvement have been suggested and are studied extensively including somatic reprogramming, trans-differentiation, paracrine signalling, and direct electrophysiological coupling.6,7 Number of in-vivo rodents and swine models has been studied as an experimental8,9 and clinical2,6 support to check the mechanism of engraftments of these cells to improve myocardial regeneration. On the basis of these rigorous studies and demonstrated safety aspects, clinical trials have also been initiated by transplanting different cell types such as skeletal myoblasts, cardiomyocytes, smooth muscle cells directly in the myocardium after which its contribution towards the angiogenesis in the infracted area and improved cardiac function is checked. Earlier to this century; source for stem cells was classified into two general categories embryonic and adult sources depending upon the time point of ontogenesis. Amongst the two embryonic stem cells pose problems related to ethical obligations, uncontrolled cancerous growth etc. Thus as an established adult stem cells source; bone marrow-derived MSCs with or without genetic modification has been extensively studied for transplantation2,10,11 before the amendment of a new updated list wherein extraembryonic tissues such as placenta, umbilical cord started getting maximum attention as more potent stem cell sources due to their hassle-free availability, lack of any ethical issues, enhanced immune tolerance.12,13
Though all fetal tissues Amniotic Membrane (AM), Wharton’s Jelly (WJ) and Villous Chorion (VC) contribute equally as a good stem cell source14−16 considered as the most attractive, hassle-free source of MSCs, mainly due to its totipotency and contribution towards maternal tolerance. These fetal-derived MSC’s are also known to be immuno-privileged thus not eliciting any immune rejection across allogeneic as well as xenogeneic barriers due to their ontogeny. In one regard they possess pluripotent characteristics of embryonic stem cells by expressing markers such as OCT-4, SOX-2 and in other regards, they are shown to express immunosuppressive markers such as HLA-G and hence are known to be immuno-tolerant. Hence, in summary, these primitive cells have a greater ability to expand in culture and have different physiology relatively because of their youth and naive status.12,17−19
In the current investigation, we made the attempt to study the differentiation potential of fetal-derived MSCs into cardiac progenitor cells in-vitro when exposed to 5’Azacytidine.20 This proposed differentiation has been confirmed with the expression of cardiac markers such as CSM1, Cardiac Troponin (cTnT). Angiogenesis capabilities of differentiated cardiac progenitors were studied further in-vitro for the formation of new blood vessels, which is essential in the growth and development of biological systems. In summary, We report that human fetal-derived MSC's displayed significant phenotypic and genotypic alteration after exposure to 5' Azacytidine by forming clusters of cardiac progenitor cells; apart from this we have also observed that the expression of angiogenic activity of fetal sources Amniotic membrane, Wharton's jelly and Villous chorion is able to deliver good, reproducible results and the opportunity to monitor angiogenic behaviour over time and therefore enabling the estimation of time-dependent effects on the neovascularization. Thus this study will put an insight into finding a safe and effective alternative approach to cell-based therapy for cardiac diseases.
Materials
Human fetal tissues AM, WJ and VC were collected from the cesarean section with parental prior approval and institutional committee approval. As per the requirement, samples were collected after the C-section in a chilled phosphate buffer saline (PBS, Himedia, India) along with Antibiotic-Antimycotic solution (15240096; Gibco, USA) and transferred to the lab immediately.
Isolation of Mesenchymal stem cells
MSCs are derived from the fetal sources Amniotic membrane, Wharton’s jelly and Villous chorion as per our previous published methodology14−16 were used for the entire study as a representative model of respective source and pooled of all three sources were used as test control (TC).
Phenotyping and purity analysis
Passage second and passage ten cells were then subjected to flow analysis for MSC specific markers such as CD90, CD105 and Hematopoietic markers such as CD34, CD45. Approximately 2x104 cells were utilized per marker. Trypsinized cells were washed with PBS to remove traces of media and TrypLE Express (12604013; Gibco, USA). Cells were collected in centrifuge tubes and incubated with each antibody or CD markers (BD Biosciences) tagged with fluorescent colors for 45 minutes at 4°C. Antibodies used were CD34 (PE), CD45 (FITC) as hematopoietic markers and CD90 (FITC), CD105 (PE) as mesenchymal markers. After incubation; (Thermo scientific, USA) tagged cells were centrifuged (Thermo scientific, USA) and suspended in PBS for analysis.
Tri-lineage differentiation
The potential for tri-lineage differentiation, i.e., adipocyte, osteocyte, and chondrocyte differentiation, of fetal-derived MSCs cultured in respective culture conditions was assessed as previously described.14−16 Images were captured using an Olympus microscope (CKX41/ CKX31; Olympus, Japan).
In-vitro myogenic differentiation
To study in-vitro cardiomyogenic differentiation potential;20 MSC’s were exposed to 5’ Azacytidine (A2385; Sigma Aldrich, UK) in 6-well plates (Costar, USA) at a density of 1x104 each with growth medium Iscoves Dulbecco’s Modified Eagle Medium (12440053; IMEM, Gibco, USA)+10% PLTMax Human platelet lysate (HPL, SCM141; Merck, Germany). After 24hours, the medium was changed to induction medium containing IMEM+10%HPL+10µM 5’Azacytidine. Incubation with induction medium was carried out for 24hours; after which medium was replaced with maintenance medium as too much exposure to 5’ Azacytidine could be harmful to cells. Maintenance medium used was IMEM/F12+2% HPL. The medium was changed every 3days for a week. Cells were observed under a microscope daily to visualize morphological changes associated with the extent of differentiation. MSC's without the addition of an induction medium were used as controls.
Tubular formation
For angiogenesis or tube formation assay21,22 Matrigel matrix (354234, BD Biosciences, Falcon) kit was used with minor modifications. Utilized 1x104 differentiated cardiac progenitors from both the sources for each well coated with Matrigel. The 4 welled matrigel plates (Corning, USA) were activated by overnight refrigeration at 4°C as per the manufacturer’s instruction. Growth medium used for the assay was Endothelial Growth Medium (EGM, Lonza, Singapore)+20% HPL and maintenance medium was EGM+2% HPL. Induction medium used was EGM+20%HPL+5ng/ml Vascular Endothelial Growth Factor (VEGF, Sigma, LLC, USA). For the first 30minutes, cells were incubated with Matrigel matrix using Growth Medium. After 30 minutes growth medium was replaced with Induction medium for 24hours followed by which cells were replaced by maintenance medium for 48hours in hypoxic conditions. Cells were observed under microscope hourly to visualize morphological changes associated with the extent of angiogenesis.
Total RNA extraction and polymerase chain reaction
Total RNA was extracted by TriZol RNA Extraction Kit (Invitrogen, USA) from differentiated cells, non-differentiated MSCs were used as negative control. Reverse transcriptase PCR of genes of cardiac transcription factors including CSM1 and cTnT were performed using 0.1ug of total RNA. The reverse- transcribed cDNAs were amplified by PCR using following oligonucleotide Primers: (a) CSM1: Forward: 5’-TGATGGAATACCGACACCAGA-3’, Reverse: 5’-GGTAGGTGGCAATCTCCACA-3’, (b) Cardiac Troponin (cTnT): Forward: 5′-GGCAGCGGAAGAGGATGCTGAA-3′ Reverse: 5′-GAGGCACCAAGTTGGGCATGAACGA-3′. cDNA was synthesized by rt-PCR kit (Quiagen, Germany). Thermal profile used for PCR was 94°C for 2 minutes followed by 35 cycles of 30 seconds at 94°C, with 30seconds annealing followed by 1-minute extension at 72°C. Additional 10 minutes’ incubation at 72°C was included after the last cycle. PCR products were size fractionated by electrophoresis using 1% agarose gel for Cardiac Markers.
Morphology and flow analysis
In about 5-7days an outgrowth of a homogenous spindle-shaped population has been observed. After about 15-21days’ cell express long, fibroblasts morphology and began to form colonies. A similar morphology was maintained throughout till tenth subsequent passaging (Figure 1). FACs analysis was employed to identify surface markers for MSC’s at second and tenth passage. Cultures were shown to be devoid of HLA-DR, CD 79a, CD 45 and CD 34, which are markers for hematopoietic cell lineage. In contrast, a very high expression of CD 105, CD 90 and CD73 was observed for all populations.
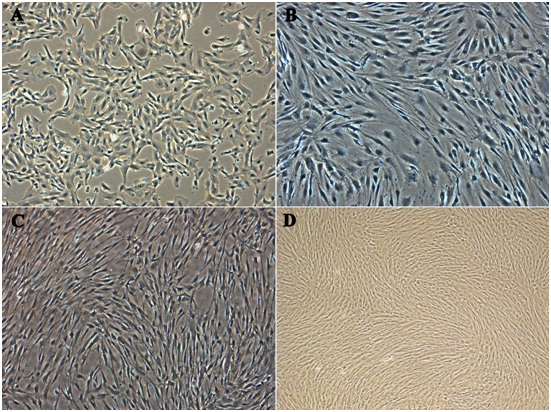
Figure 1 Morphological analysis of Multipotent Stromal Cells. The growth of the cells after 24 hours of incubation at passage 10, at which time the cells were mostly fibroblast-like cells in shape. Comparison of the morphology: (A) Amniotic membrane, (B) Wharton’s jelly, (C) Villous Chorion, (D) Complete confluence growth of pooled cells as test control respectively (Magnification 10X).
Tri-lineage differentiation
There was no visual difference in multilineage differentiation capacity of fetal-derived MSCs towards adipogenic, chondrogenic and osteogenic lineages in all different culture conditions tested in the experiments. As evident from Figure 2; all different fetal source MSCs retained their capacity to differentiate into adipocytes, chondrocytes, and osteoblasts. For adipogenic differentiation, small fat droplets in the cytoplasm could be observed after 21days of induction (Figure 2; AM-A, WJ-A, VC-A). Induced cells morphology gradually became larger and expanded. Lipid droplets that stained with oil red accumulated in the cytoplasm of positive cells, and the number of stained cells increased in a time-dependent manner, and no fat droplet was detected in the control. For osteogenic differentiation of WJ and VC, cells started to change morphologically as early as 7 days in an inducing culture medium. The cells lost their typical fibroblast appearance and showed a rounder, more cuboidal shape. The ability of VC-MSCs to differentiate into osteocytes was grown under osteogenic differentiation conditions acquired calcium accumulation was assessed by von Kossa staining; the intense black staining was detected between osteogenic cells (Figure 2; WJ-O, VC-O), and calcium deposition was not observed in cells cultured under control conditions. For AM, Induction of osteogenesis in the expanded MSCs resulted in extracellular matrix mineralization which was confirmed by Alizarin Red staining (Figure 2; AM-O). To confirm the chondrogenesis potential of the WJ and VC-MSCs, positive staining of expanded collagen fibers stained with safranin O (Figure 2; WJ-C, VC-C), and positive staining of AM-MSCs collagen fibers with alcian blue indicated that MSCs were successfully differentiated to chondrocytes (Figure 2; AM-C). No change

Figure 2 Tri-lineage differentiation of representative fetal sources, Amniotic membrane (AM), Wharton’s jelly (WJ) and Villous Chorion (VC) MSCs retained their capacity to differentiate into Adipocytes (A), Osteoblasts (O) and Chondrocytes (C) respectively (Magnification 10X).
Myogenic differentiation
As postulated; MSC’s were subjected to differentiation into cardiac progenitor cells after exposure to 5’ Azacytidine. Cells were observed 24hours post exposure; some of the cells died and started floating on the surface of media. Surviving cells began to proliferate and differentiate altering morphology after approximately 6-7 days in a culture media. Cells were begun to undergo characteristic cardiac progenitor morphological changes from fibroblast-like to round ball like light emitting cells (Figure 3). These cells were further analyzed for cardiac markers through rt-PCR cardiac-specific markers.
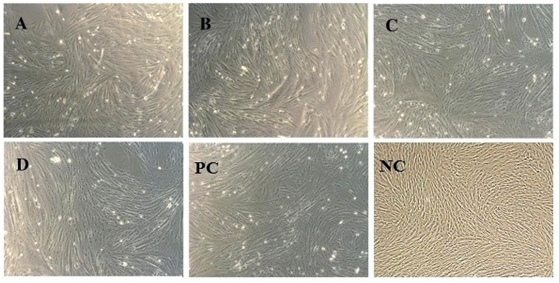
Figure 3 In-vitro Myogenic differentiation. Morphological changes of MSC’s from fibroblast to round ball like light emitting cells after exposure to 5’Azacytidine; PC: Positive control and NC: Negative control (Without exposure to 5-Azacytidine) A, B, C, D: AM, WJ, VC and test control representing respectively (Magnification 10X).
PCR analysis
Total RNA from induced and non-induced cells was isolated and analyzed by rt-PCR. It has been observed that no expression of cardiac-specific genes was detected in untreated MSC's, but 5' Azacytidine treatment strongly induced expression of cardiac transcription factors including CSM1 and cTnT. As shown in (Figure 4); these 5’ Azacytidine induced MSC’s; expressed cardiac markers comparable with known molecular weight marker 207, 150 base pairs respectively when run on an agarose gel. Total RNA obtained from non-induced MSC’s was treated as negative control.
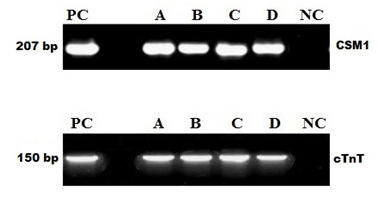
Figure 4 5'Azacytidine induced cardiac-specific genes CSM1 and cTnT. Expression of CSM1 and cTnT genes were analyzed by RT-PCR; Lane PC: Molecular Marker; Lane A, B, C, D: AM, WJ, VC and test control respectively, Expression of CSM1 and cTnT in 5’ Azacytidine treated samples comparable with PC; Lane NC: No expression of cardiac-specific genes was detected in untreated MSC.
Expression of angiogenic activity
Capillary network formation a distinct mesh-like structure is observed when differentiated WJ-MSCs were cultured and seeded on Matrigel and exposed to VEGF. After around 1-2hour WJ-MSCs started migrating and began to align themselves to form in a spheroid form and tube-like structure or tubule formation was observed post-three-hour incubation. About 11-14hour later closed polygonic structure began to appear which turned to the complex mesh-like structure within the next 24hours (Figure 5; WJ-S and WJ-T). However, in case of AM-MSCs (Figure 5; AM-S and AM-T) and VC-MSCs (Figure 5; VC-S and VC-T) not clear network formation is observed but tubule formation in a spheroid form was observed, when cultured on matrigel; AM-MSCs and VC-MSCs isolated from different donors manifested varying degrees of tubule formation at 16hours. In addition, MSCs spheroids demonstrating the greatest cumulative branch length and the total number of branches of the respective samples. No changes were observed in the untreated control (Figure 5; AM-C, WJ-C, and VC-C).
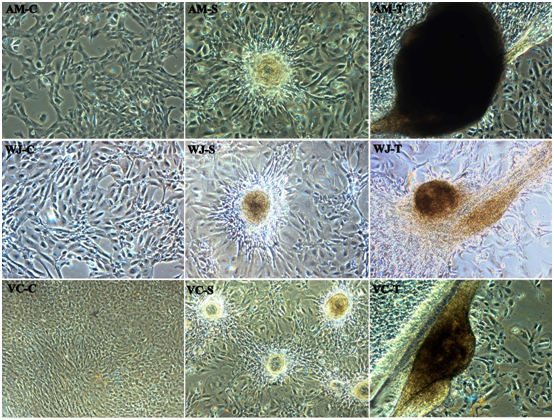
Figure 5 In-vitro Tube formation of representative fetal sources Amniotic membrane (AM), Wharton’s jelly (WJ) and Villous Chorion (VC). No changes in the control (C) and alignment of cells started one-hour post incubation in a Spheroid form (S), Extensive network formation / Tubule formation post-three-hour incubation (T) respectively (Magnification 10X).
Regenerative properties of MSCs has progressed rapidly from the budding concept as bystanders of uncertain significance to the essential cells governing tissue homeostasis by regulating niches.9 This unique property along with the capacity for multipotential differentiation has been greatly appreciated as their therapeutic potential. For nearly a century, the adult heart was viewed as a terminally differentiated post-mitotic organ; characterized by a predetermined number of cardiomyocytes established at birth and preserved throughout the life of an organism. These cells are known to be responsible for normal wear and tear of the body and thereby contributing towards the successful functioning of the heart. But some external factors such as age, shock etc may account for the significant loss of these cells which ultimately can cause impaired functioning of the tissue leading to infarction. Ultimately myocardial Ischemia and cardiac diseases are responsible for major worldwide death toll and hence researchers are struggling to find out newer therapeutic alternatives over the conventional treatment options due to their skeptical efficiencies. One of the underlying objectives in cardiac regenerative therapies may be a replacement for damaged or lost resident cardiomyocytes to improve heart function.23−27
Evidence in the last decade has clearly established the beneficial effects of fetal-derived MSCs such as their ease of isolation, differentiation potential, and immunoprivilege. The early demonstrations of these effects, coupled with the remarkable safety profile, have set the stage for pivotal testing of these cells as a therapeutic cell-based alternative. Data obtained from many clinical and preclinical studies demonstrated that MSCs isolated from fetal tissues exhibit a different healing performance in cardiac regeneration. There are also evidences that MSCs isolated from fetal sources exhibiting some pluripotent properties showed a favourable survival pattern in infarcted hearts. Some studies depicted the crucial role of some of the cytokines and factors for homing of endogenous and exogenous stem cells early after myocardial infarction and ultimately resulting in some advancement inefficient myocardial engraftments.28−33
The current study addresses the effect of 5' Azacytidine on an in-vitro differentiation of fetal MSC's into cardiac progenitor cells and confirmed that they differentiated successfully in response to the stimuli. Morphological observation of all cultures and subcultures showed long spindle-shaped fibroblast 4-5days after isolation. The mesenchymal origin of isolated cells was confirmed with Flow analysis and tri-lineage differentiation. Approximately more than 90% population was positive for the mesenchymal marker and negative for hematopoietic markers. All cells subjected to tri-lineage differentiation attained remarkable morphological difference associated with the particular origin. Since data collected showed that MSCs transdifferentiate into cardiomyocytes in-vitro, we enriched a pool of fetal-derived mesenchymal stromal cells, which we subjected to myogenic differentiation by exposing cells to 5'Azacytidine. After exposure initially some cells were observed to be dead and started floating on the surface. Whereas, adhered cells continued to multiply and with the increased exposure time developed blunt spherical light emitting morphology. This could be due to 5' Azacytidine, at high concentrations, is highly toxic to cultured cells34−35 and, 5' Azacytidine has been reported to cause DNA demethylation, gene activation, nonspecific transcription, and translation.35−37 However, recently, this demethylation and protein synthesis mechanism in 5' Azacytidine induces cell toxicity has been the subject of controversy.38 Even, 5’Azacytidine is lethal at high concentration but it is also a differentiation inducer so that might be the reason for cytotoxicity but the cells which resisted can able to differentiate into tube formation and help in cardiac repair. Notably, these cells when subjected to reverse transcriptase PCR were observed to express some cardiac-specific genes including CSM1 and cTnT. The results confirmed that fetal-derived MSCs were successfully able to differentiate to cardiac progenitor cells with response to of 5' Azacytidine.
Migration of vascular endothelial cells plays a significant function in vasculogenesis and angiogenesis.39 Silva et al.40 revealed that MSCs intervention into ischemic myocardium can differentiate into endothelial cells and smooth muscle cells in-vivo, resulting in improved vascularity and cardiac functions.40 Differentiation of MSCs into endothelial cells in-vitro did not improve their performance in-vivo.41 Since, in this study fetal-derived, MSCs were studied to differentiate into adipogenic, chondrogenic and osteogenic lineages, they can also be considered as a promising source for obtaining endothelial cells that are able to generate vascular networks. Precisely, the formation of new blood vessels is essential for the growth and development of biological systems. The in-vitro tube formation assay is based on the differentiation of endothelial cells and the formation of tube-like structures on an extracellular matrix. Thus, this assay proved that the expression of angiogenic activity of fetal sources Amniotic membrane, Wharton's jelly, villous chorion, and pooled cells are able to deliver good, reproducible results.
Though the current in-vitro study showed the possible potential benefits of fetal MSCs to improve cardiac repair. Hence, it is necessary to continue undertaking adequately designed pre-clinical studies and clinical trials provided that all the three different sources experimental grounds are robust and consistent. In addition to this, the pooled stem cells also should be selected considering their success in a clinical application would give a solid foundation for the further development of regenerative medicine based on cell therapy. Further work is thus needed to confirm the proof of principle, to evaluate potential middle level and long-term safety issues. Thus, these stem cells may be used both in the regeneration of damaged tissue and to create ex-vivo organ fragments in the future.
We wish to thank directors Lalit Jaiswal, Firdosh Mahuvawalla and Kangan Jaiswal of Cellu Gen Biotech Pvt. Ltd. for the necessary support for the study.
Authors declare that there is no conflict of interest.

©2018 Kannaiyan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.