Journal of
eISSN: 2373-4426


Case Report Volume 13 Issue 2
Department of Pediatric Surgery, Royal Hospital, Sultanate of Oman, Oman
Correspondence: Dr. Lalith Wijesinghe (Main Author), FRCS (Glsg), FEBPS, Senior Specialist, Pediatric Surgical Department, Royal Hospital, PO box 1331, Code 111, Muscat, Sultanate of Oman Specialist, Oman, Tel 96892718900
Received: May 10, 2023 | Published: May 31, 2023
Citation: Wijesinghe L, Maji DC, Shaker N, et al. Unilateral pulmonary agenesis, esophageal atresia, and tracheo esophageal fistula: no more a fatal condition. J Pediatr Neonatal Care. 2023;13(2):106-111. DOI: 10.15406/jpnc.2023.13.00500
The occurrence of unilateral pulmonary agenesis (PA) and esophageal atresia with and without tracheo esophageal fistula (EA/TEF) is exceedingly rare. The common origin of the esophagus and the lungs from the primordial foregut explains this association. This study reports on the accuracy of the preoperative investigations, operative approach, and a review of the literature and our own series.
Keywords: esophageal atresia, tracheo-esophageal fistula, unilateral pulmonary agenesis
Esophageal atresia/tracheoesophageal fistula (EA/TEF) is a condition resulting from abnormal development before birth of the tube that carries food from the mouth to the stomach. There are several types of esophageal aresia, classified by the location of the malformation and the structures that are affected. In more than 80 percent of cases, the lower section of the malformed esophagus is connected to the trachea (EA with a distal TEF). Other possible configurations include having the upper section of the malformed esophagus connected to the trachea (EA with a proximal TEF), connections to the trachea from both the upper and lower sections of the malformed esophagus (EA with proximal and distal TEF), an esophagus that is malformed but does not connect to the trachea (isolated EA), and a connection to the trachea from an otherwise normal esophagus (H-type TEF with no EA). EA/TEF occurs alone (isolated EA/TEF) in about 40 percent of affected individuals. In some cases it occurs with other birth defects or as part of a genetic syndrome (non-isolated or syndromic EA/TEF). EA/TEF occurs in 1 in 3,000 to 5,000 newborns.
The occurrence of both unilateral PA in combination with EA/TEF in a patient is exceedingly rare.1–15 The common origin of the esophagus and the lungs from the primordial foregut explains this association.2–16 It was first described by Paul in 19294 and only 25 such cases have been reported in the literature, during the last 30 years.1–15 It was believed to be a fatal combination, even in the absence of other life threatening defects.1–3,6,8,9,11,12 There were no reported survivors of early primary repair in the neonatal period.3,6
During the last 3 decades, the treatment strategy has evolved favoring the early primary repair.1,2,6,10,14,17,18 The first reported survivor is from 1978 in the Cleveland series.2 This retrospective study aims to report this rare combination of anomalies among EA/TEF patients. We report on three patients with unilateral PA and EA (two with TEF and one without) and their preoperative investigations, operative managements are described.
It is a retrospective study of cases done in Royal Hospital, Muscat, a tertiary referral center of the Sultanate of Oman between January 1985 to December 2012. Institutional approval for publication of the case was obtained from Dr Mohammed Jaffar Sajwani FRCS, the Head of the Department as per protocol and policies prevalent.
The operation theatre registry of Khoula hospital and Royal hospital were used to identify the EA/TEF patients treated over the last 27 years (1985 to 2012). The patients with EA/TEF with unilateral PA were identified and their case notes were retrospectively reviewed. The birth weight, gestational age, associated anomalies, preoperative investigations, surgical management and long-term prognosis are reported.
This retrospective review (from 1985 to 2012) identified 219 patients with EA/TEF. Three patients had associated PA. Each patient detail is described below.
Patient 1
A term baby boy, with a birth weight of 2.89 Kg, was transferred to us on Day three of life as he had choking upon starting feeds with respiratory distress and an attempt to insert nasogastric (NG) tube had failed.
On presentation the baby was stable on nasal Oxygen. The chest x-ray showed a completely opaque right (Rt.) hemi thorax, a shifted mediastinum to the Rt. with dextrocardia, a NG tube coiled at T 2 vertebral level and a few cleft vertebrae in upper and mid thoracic level (Figure 1.1). He was taken up for Rt. thoracotomy, ligation of the fistula with primary esophageal anastomosis. Neither Rt. lung nor Rt. bronchus were visualized and the shifted heart and major vessels made the procedure difficult. The lower esophageal pouch and the TEF were found to the left (Lt.) side of the distal trachea with a fairly wide gap of five cm between the upper and lower esophageal pouches; therefore, the primary anastomosis was carried out with some tension. Post-operative recovery was uneventful except for the ventilator requirement. The ultrasound scan (US) of the head was normal and the echo cardiogram revealed a dextraposed heart with an aplastic Rt. pulmonary artery associated with hypoplastic lung.
At the age of three weeks, a water soluble upper gastrointestinal contrast study revealed a mild stricture at 13 cms. It was dilated endoscopically at the age of two months and three months. The follow up contrast study at five months of age revealed normal esophageal lumen. However there was a smooth compression externally on the posterior aspect of the esophagus, below the level of the carina. A computer tomography (CT) and a CT angiogram confirmed the agenesis of the Rt. lung, absence of Rt. pulmonary artery and dextraposition of the heart with displaced mediastinal structures while excluding a vascular ring (Figure 1.2 & 1.3).

Figure 1.1 Chest X-ray.
H: completely opaque Rt. hemi thorax, M: ipsilateral mediastinal shift, C: apparent dextrocardia, L: hyperinflation of contra lateral lung, O: coiled NG tube

Figure 1.2 CT chest (lung window).
A: absent Rt. Lung, B: Lt. upper and lower lobe bronchi, C: dextroposition of heart - displaced in to the Rt. Hemi thorax, L: hyperinflation of contra lateral lung
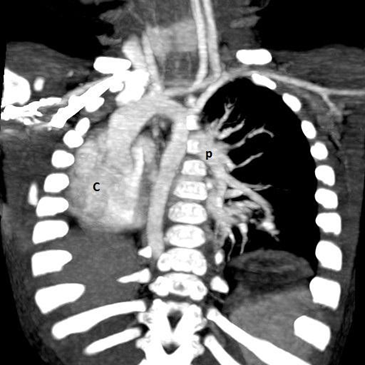
Figure 1.3 CT angiography (reconstructed 3D).
P: Lt. pulmonary artery and branches, C: dextroposition of heart – displaced in to the Rt. Hemi thorax
He was last seen at the age of 24 months in reasonable health.
Patient 2
A term, 2 Kg, baby girl with a poor Apgar, had to be bagged at birth and was shifted to special care baby unit where excessive oral secretions were noted. The x-ray of the chest revealed a coiled NG tube, positive gastric air and complete opacity in the Lt. hemi thorax with a shift of the mediastinum to the Lt. side (Figure 2.1).
She was transferred to our institution on day two of life with a provisional diagnosis of EA/TEF and a collapsed Lt. lung.
On arrival the baby was stable with normal oxygen saturations. The auscultation of the chest revealed diminished air entry on the Lt. lung field and a cardiac murmur.
At Rt. thoracotomy, a Rt. side aortic arch obscuring the shifted trachea and esophagus made the operative procedure difficult (Figure 2.2).
The ligation of the TEF and the esophageal anastomosis were carried out. Postoperatively, the baby was ventilated for seven days and she made an uneventful recovery except for a brief episode of aspiration pneumonia.
The associated anomalies included a short neck, humped up shoulders, abducted hips, flexion deformity of the Lt. wrist, six lumbar vertebrae, three sacral vertebrae and an absent coccyx. The echo cardiogram revealed an absent Lt. pulmonary artery with severe pulmonary artery hypertension (PAH) and a ventricular septal defect. The baby was treated for heart failure. The US of the renal tract was essentially normal albeit somewhat small kidneys.
The CT scan of the chest showed Lt. upper lobe agenesis, absent Lt. pulmonary artery, a double aortic arch (Rt. being the main) and an aberrant vessel from the Lt. aortic arch supplying the Lt. lower lobe of the lung (Figure 2.3).
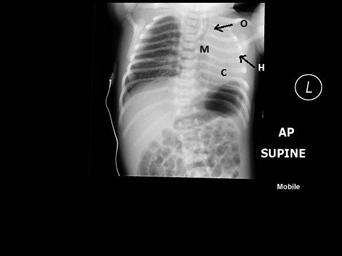
Figure 2.1 Chest X –ray.
H: completely opaque Lt. hemi thorax, M: ipsilateral mediastinal shift, C: apparent Dextrocardia, L: hyperinflation of contra lateral lung, O: coiled NG tube
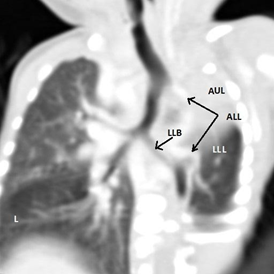
Figure 2.3 CT chest (reconstructed).
AUL: absent Lt. upper lobe, LLB: Lt. Lower lobe bronchus, ALL: aberrant artery from the aortic arch supplying Lt. lower lobe, L: hyperinflation of contra lateral lung AA1: aortic arch 1, AA2: aortic arch 2.
A subsequent bronchoscopy for recurrent chest infections and choking episodes at the age of five months, revealed a very short tracheal bronchus from the Lt. Side of the trachea with rudimentary lung tissue. The Barium swallow that followed showed a tight anastomotic stricture, which was managed by several endoscopic dilatations. A transgastric transpyloric feeding jejunal tube was inserted.
At nine months of age, a CT angiogram confirmed the Lt. Upper lobe agenesis, absent Lt. pulmonary artery and the aberrant artery from the Lt. aortic arch to the Lt. lower lobe (Figure 2.2 & 2.3). She underwent re-implantation of the aberrant vessel in to the main pulmonary artery successfully by the cardio-thoracic surgeons. A few months later a Nissen fundoplication and a gastrostomy was carried out for resistant gastro-esophageal reflux. She is four years old now, and the last admission was three months back for formal gastrostomy closure as it had failed to close spontaneously. She is tolerating the normal diet and is gaining weight well.
Patient 3
A 2 kg, baby girl of 34 weeks gestation, born to a multigravida mother with an antenatal history of polyhydraminios, was noted to have frothy secretions at mouth. The inserted NG tube was stuck at the pharynx hence the transfer to our institution on day one of life with a diagnosis of EA/TEF. Clinically apart from the pure EA, she was noted to have an anterior ectopic anus along with absent Rt. First metatarsal bone. The chest and abdominal x-rays showed a completely opaque Rt. chest with an upper esophageal pouch ending at cervical seven vertebra level and complete absence of bowel gas in the abdomen indicating a pure atresia with a wide gap. The plain films also revealed 14 ribs on the Rt. side, 13 on the Lt. side and a partial sacral agenesis. The echocardiogram showed a VSD. The conglomeration of abnormalities noted conformed to the VACTERL (Vertebral, Anal, Cardiac, Tracheo-esophageal fistula, Renal and Limb defects) association.
She underwent laparotomy and gastrostomy on the same day. She was ventilated for a few days and was managed by a regime of frequent upper pouch suctioning and gastrostomy feeds. The US of the renal tracts was normal. The CT of the chest a few months later showed the completely opaque Rt. chest occupied by the heart, the absent Rt. lung with a very short Rt. bronchus confirming the Rt. pulmonary agenesis.
At the age of four months after a reasonable weight gain, she underwent trans-hiatal colonic interposition uneventfully. Once she became confident with the oral feeds the gastrostomy tube was removed. She is on regular follow up. She had four admissions over the next five years with sub-acute intestinal obstruction, all of which were managed successfully by non-surgical management.
At the age of eight years, she was referred to the Gastroenterologists for endoscopy of the pulled up segment, to rule out any possibility of metaplastic changes. Currently a six monthly Gastroenterology follow up has been established.
This year she celebrated her 11th birthday.
Even though In 1696, Gibson provided the first description of esophageal atresia with a distal TEF, it was only In 1939 and 1940, Ladd of Boston and Lever of Minnesota first achieved surgical success in stages. The treatment plan for each baby must be individualized. Prognostic classifications (eg, the Waterston, Spitz, and Poenaru prognostic classification systems) can provide guidance in patients with multiple problems and help determine the indications for and timing of surgical repair, but early and decisive identification of the most life-threatening anomaly is essential. Surgical approaches to treatment vary according to surgeons' preferences and variations in pathologic anatomy.
The indiscriminate use of the term PA has given rise to some considerable confusion prior to the 20th century. It was put to rest in 1912 by Schneider and finally by Boyden in 1955 with a classification based on the degree of the developmental arrest16 which should be adhered to, in reporting new cases (Table 1).
Group 1 - AGENESIS: absent lung, no bronchus |
Group 2 - APLASIA: absent lung, rudimentary bronchus |
Group 3 – HYPOPLASIA: l obar agenesis, hypoplastic lung tissue, normal bronchus |
Table 1 Boyden classification of pulmonary agenesis16
Our three children are represented in each group. The first baby is in Group 1: Agenesis having no Rt. lung, no bronchus nor a Rt. pulmonary artery. The second baby with only Lt. upper lobe agenesis falls into Group 3: Hypoplasia. The last baby with no Rt. lung but with a short rudimentary bronchus and no functioning alveoli represents the Group 2: Aplasia. Twenty five babies of EA combined with PA have been reported during the last 30 years1–15 (Table 2).
|
No |
Reference |
Anatomy |
Anomaly |
Surgical approach |
Outcome |
||
|
PA |
EA |
TEF |
Repair |
Alive/ Expired |
|||
|
[1] |
Bereton RJ… 1983 |
Rt. Gp. 1 |
EA |
wide |
N |
Staged repair |
Expired at 2m |
|
Rt. Gp. 1 |
EA |
TEF |
CHD |
Early primary repair |
Expired at 2d |
||
|
[2] |
Benson JE… 1985 |
Rt. Gp. 2 |
EA |
TEF |
V |
Early primary repair |
5y |
|
Rt. Gp. 1 |
EA |
TEF |
V |
Early primary repair |
7y |
||
|
Rt. Gp. 3 |
EA |
TEF |
Sy. |
Early primary repair |
7m |
||
|
Rt. Gp. 3 |
N |
BEF |
N |
Early primary repair |
1y |
||
|
Rt. Gp. 3 |
EA |
TEF |
V |
Early primary repair |
3m |
||
|
Rt. Gp. 3* |
EA |
BEF |
V |
NS |
Expired at 23h |
||
|
Rt. Gp. 3 |
EA |
TEF |
V |
Early primary repair |
4y |
||
|
[3] |
Preston R… 1986 |
Rt. Gp. 2 |
EA |
TEF |
O |
Staged repair |
Expired at 10m |
|
[4] |
Takayanagi K… 1987 |
Rt. Gp 1 |
EA |
TEF |
V |
Staged repair |
2m |
|
[5] |
Ambroise MJE… 1988 |
Rt. Gp. 1* |
EA |
TEF |
N |
NS |
Expired at 24h |
|
[6] |
Hoffman MA… 1989 |
Rt. Gp. 1 |
EA |
TEF |
N |
Early primary repair |
2y |
|
Rt. Gp. 1 |
EA |
TEF |
DA |
Early primary repair |
1.5y |
||
|
[7] |
Iyengar Jk… 1991 |
Lt. Gp. 2 |
EA |
TEF |
V |
Early primary repair |
Expired at 25d |
Table 2 Summary (2) of previously reported 25 cases1–15
*autopsy findings, BEF-bronchoesophageal fistula, CHD-congenital heart disease, DA-duodenal atresia, d-days, hrs-hours, m-months, N-none, NA-not available, NS-no surgery, O-other, Sy-syndromic, yrs-years , V- VACTERL
About 50% of them had true PA (Group 1), which also is the commonest form. Another 25% was found with Hypoplasia (Group 3). Two of our babies are girls. The review of published literature suggests a slight preponderance in the female gender (3:2). More than 50% babies were born prematurely and the majority had a birth weight ranging from 1-2 kg. A positive antenatal scan with polyhydramnios was reported in three cases but no data was available in all the other patients .In our case series only one patient had polyhydramnios.
Although the isolated PA is found most commonly on the Lt. side,19 when associated with EA, a remarkable dominance in the incidence on the Rt. side is noted.2 In the 25 published cases, all but one had Rt. sided involvement of the lung.7 In our series two had Rt. PA. Interestingly though, there had been several Lt. sided PA reported prior to the three decades under review. One series has seven out of eight cases of Lt. sided agenesis with only a TEF (H type) in the absence of EA.2 Although this pattern was seen neither in the 25 published cases nor in our three children, a single case of a broncho-esophageal fistula without EA in a baby with Rt. PA has been reported.2 Apart from this, all but 2 patients in the published series had EA accompanied by TEF where as one out of our three patients had a pure EA.
There are many modalities used in the diagnosis of pulmonary agenesis. The clinical findings of absent breath sounds and a shifting apex beat on auscultation, together with the findings of completely opaque hemi thorax, ipsilateral mediastinal shift in a plain x-ray film, are enough to arouse one’s suspicions of PA. These were consistently seen in the case review and most of them were found in our three babies as well. Apart from that in a quarter of patients in the case review, an apparent dextrocardia and an over inflated contra lateral lung too were noted.
Of the 25 cases under review, in about 50%, the confirmation of PA was made at the time of operation and it was further substantiated by a CT scan of the chest, or a bronchoscopy / bronchography. Few of them were identified at autopsy. A CT angiogram or an echocardiogram was used to identify the absent ipsilateral pulmonary artery to support the diagnosis of true agenesis or aplasia.16 In a few instances an angiocardiogram and a ventilatory perfusion scan had also been used. The majority of the cases in the review had only one modality other than the plain x-ray chest film for the final confirmation. A quarter of them had carried out two or more modalities (Table 3).
|
X-Ray Chest: completely opaque hemi thorax ipsilateral mediastinal shif apparent dextrocardia hyperinflation of contra lateral lung |
25 |
|
Echo Cardiogram: absent pulmonary artery |
4 |
|
Bronchoscopy/Bronchography: blind ended main bronchus |
8 |
|
CT Chest/ CT Angio: completely opaque Hemi Thorax absent/rudimentary bronchus absent pulmonary artery |
5 |
|
Angio Cardiogram: absent pulmonary artery |
2 |
|
Lung scan: absent ventilation & perfusion |
1 |
Table 3 Imaging modalities used in the diagnosis of PA in all the 25 cases in the literature review1–15
In our series of three children, the x-ray chest showed the typical findings (Table 3) and in the first two patients the absence of lung was confirmed at the primary operation, while a subsequent bronchoscopy, performed to find the cause of the recurrent chest infections reconfirmed it in the second child. In the case of the third patient, a CT chest confirmed the involvement of the lung. The echocardiogram done in all our children helped to detect the status of the ipsilateral pulmonary artery and the CT angiogram reconfirmed it subsequently.
It is well known that even in isolation, both EA/TEF and unilateral PA carry around 50% chance of having other associated anomalies, the majority being the VACTERL sequence.3,4,11 Out of the 25 cases in the review 64% had other anomalies, 32% of which belonged to VACTERL group. While another 32% had no other anomalies, in 4% of the reviewed cases, it was unknown. An association with duodenal atresia was noted in five cases (Table 2). One patient had a duplicated azygous vein. In our three patients while none were associated with neither azygous vein anomalies nor DA, VACTERL sequence was noted in two.
The accepted management for this condition until the 1980s and even up to the late nineties3,4,8,9,12 was the staged repair with an initial gastrostomy and delayed repair. It is still practiced when a primary esophageal repair cannot be done because of an excessively wide gap. The concept of early primary repair became popularized in mid 1980s1,2,6 despite the fact that no survivors had been reported. The emergence of advanced neonatal care and improvement in the surgical techniques and management may have played a major role in the success of the primary repair.17 The early primary repair is the current treatment for this combination unless it is technically not feasible.10,14
In the last three decades, 13 out of 22 cases that made it to surgery were treated by early primary repair and eight survived giving a success rate of 62%. Four of them had Group 3: Hypoplasia, another three with Group 1: Agenesis and the last patient were in Group 2: Aplasia. The early gastrostomy, with delayed repair was performed in 9 babies and six of them survived (67%).Thus there is no significant difference between the primary repair and staged repair groups. Most patients would require additional procedures like fundoplications or correction of any vascular anomalies at one stage or another. The longest survivor of the staged repair was two and half years old. Five of this group had true Agenesis (Group 1) and the remaining case was with Aplasia (Group 2).The longest survivor who was seven years old at the time of publication came from the group that underwent early primary repair (Tables 4 & 5).
|
|
Total |
Agenesis |
Aplasia |
Hypoplasia |
|
Total |
25 |
13 |
4 |
7 |
|
Died pre operatively* |
3 |
1 |
1 |
|
|
Died post operatively |
8 |
4 |
3 |
1 |
|
Surviving at publication |
14 |
8 |
1 |
5 |
Table 4 Outcome based on the type of PA 25 cases of EA/TEF with PA diagnosed between 1983-20121–15
*autopsy in 2 patients only
|
|
Early primary repair |
Early Gastrostomy & delayed Repair |
|
Total |
13 |
9 |
|
Dead |
5 |
3 |
|
Alive at publication |
8 (62 %) |
6 (67 %) |
|
Longest Survivor at Publication |
7yrs |
2½ yrs |
The protection of the pulmonary units should be the prime goal in the management3,6,8,10 of these babies which is best achieved by an early restoration of the esophageal continuity and the division of the fistula unless it is technically not feasible at the initial operation. In that situation a cervical esophagostomy is constructed to ensure maximum function of the already compromised healthy lung volume.6
We had performed an echocardiogram and CT angiogram in all our patients where the initial x-rays had shown the features of PA in cases of EA or EA/TEF. This helped us to plan novel procedures to salvage the lower lobe which had normal airways and abnormal systemic arterial supply without a pulmonary artery as in case number 2. Removal of this lobe would have resulted in pneumonectomy and its attendant long term sequel. At 4 year follow up the child is doing well and the lungs are well expanded. We are yet to perform a VQ scan which will be done in due course.
In our institution, we perform the early primary repair for all the patients with EA/TEF with PA of all groups, unless the type of EA dictates otherwise, as in our patient 3. There was a very wide gap without a TEF and therefore it was necessary to perform an early gastrostomy and a delayed repair.
While either EA or PA in isolation is compatible with long term survival into adulthood, in combination they were believed to be uniformly lethal in the past.3,6,8,9,12 It is now no longer considered to be incompatible with life.1,8,9 The reported follow up in the literature review of the 25 published cases in the last 30 years is inconsistent and the long term outcome of survivors is unknown. Nevertheless it has shown that 36% have survived their first birthday, another 20% have celebrated their second year in life and 2 children2 have passed beyond the age of five years. One of them reached seven years making him the longest survivor published (Table 5).
All our children have survived one, four and 11 years respectively and to the best of our knowledge, patient 3 with PA is the world‘s longest survivor known, entering her second decade of life this year. Although the PA with EA/TEF along with other major co-morbidities like prematurity, major cardiac defects etc undoubtedly contribute to the morbidity and to the mortality,1–2 the long term survival is still a strong possibility. Certainly, better treatment planning together with longer and better follow up will improve our knowledge of this condition further.
None.
None.
The authors declare that they have no conflicts of interest.

©2023 Wijesinghe, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.