Journal of
eISSN: 2373-4426


Research Article Volume 13 Issue 2
1Former Research Scholar, Department of Anthropology, University College of Science, Technology & Agriculture, University of Calcutta, India
2Associate Professor, Department of Anthropology, University College of Science, Technology & Agriculture, University of Calcutta, India
3Assistant Professor, Department of Anthropology, Dinabandhu Mahavidyalaya, India
4Professor, Department of Anthropology, University College of Science, Technology & Agriculture, University of Calcutta, India
Correspondence: Dr. Piyali Das, Assistant Professor, Department of Anthropology, Dinabandhu Mahavidyalaya, Bongaon-743235, West Bengal, India, Tel +919433435924
Received: May 30, 2023 | Published: June 5, 2023
Citation: Dey B, Chatterjee D, Das P, et al. Sexual dimorphism of papillary number (PN) and main line index (MLI) among autistic patients of West Bengal, India. J Pediatr Neonatal Care. 2023;13(2):113-115. DOI: 10.15406/jpnc.2023.13.00501
Dermatoglyphic traits area well-known clinical marker for several congenital malformations and neurodevelopment disorders. The dermatoglyphic patterns and neural tissues originate from the ectodermic layer within the first and second trimesters of intrauterine life and are unaltered throughout an individual's life. Autism is a complex neurodevelopmental disorder characterized by significant disturbances in social-communicative and behavioral functioning. To the best of our knowledge, the present study is the first attempt to understand the relationshipbetween Papillary Number (PN) and Main Line Index (MLI) as an assessment of Sexual Dimorphism among the Autisticpatients from Bengalee Hindu caste population of West Bengal, India. To achieve this purpose, bilateral palm prints of 100 (67 males and 33 females) diagnosed Autistic patients and 100 (55 males and 45 females) healthy controls without any family history of Autism have been collected from the Bengalee Hindu caste population of West Bengal. All the prints were collected by the standard Ink and Roller Method (Cummins & Midlo, 1961).The result demonstrated that significant (p<0.05) sexual dimorphism on the PN and MLI was found between the Autistic males and Autistic females combing both hands. Nevertheless, the controls never showed any sexual dimorphism in the context of PN and MLI. Therefore, the present study suggests that the sexual dimorphism of the PN and MLI among Autistic patients might be other beneficial dermatoglyphic traits for Autism detection.
Keywords: dermatoglyphics, papillary number, main line index, sexual dimorphism, autism
Dermatoglyphics is the classification and measures of the cutaneous ridges on the volar side of fingers, palms, and soles, formed during the 7th-24th week of intrauterine life.1 Dermal ridges and ridge patterns are highly heritable, durable, and age-independent human traits. Neural and dermal tissues share some aspects of development, such as similar ectodermal origin, rapid development during the second trimester of gestation, and susceptibility to neuronal growth factordermatoglyphic variations are informative for early developmental brain disturbances.2–4 Many studies have resorted to dermatoglyphic variables as a valuable marker of an adverse intrauterine experience.5 Specifically, simplification, ridge dissociation, abnormal features, and fluctuating asymmetry (FA) of dermatoglyphics have been analyzed in the study of psychiatric disorders.6 A relationship exists between embryonic stress and distortion of dermatoglyphic patterns7 as a claim, several dermatoglyphic investigations have been carried out by several researchers all over the world to find out the relation of unusual dermatoglyphic traits with genetic as well as chromosomal aberrations like Trisomy 21 or Down’s syndrome,8–12 Turner’s syndrome,13,2 Klinefelter’s syndrome,14 Fragile X syndrome,15 Schizophrenia16 and E-β thalassemia.17–19 Autism is a complex brain condition that presents at around age two with a core set of symptoms, including unusual ways of relating to people, language development and delay, and repetitive or stereotyped behaviors.20 The prevalence rate is reported in India as 1 in every 250 live births, with a 4:1 male-female ratio.20,21 The modern genetic and bio-medical investigation suggested that Autism was an outcome of the chromosomal rearrangements on the long arms of chromosomes 2q, 7q, 16p, 19p22 and 15q.23,24 A Denmark-based case study reported that an extra satellite presumptive acrocentric or an isochromosome led to infant Autism in a developmentally disabled girl.25 Apart from the structural alteration of the chromosomes, several Autism susceptibility loci within the long distal arm of chromosome 7q,24,26–28, 22q24,28,29 and X-linked Neuroligins30 were linked with the server language and socialization deficits on Autism. Having a robust genetic etiology, several dermatoglyphic investigations have been carried out in Autism.25,31–35
To achieve the present purpose, 100 (67 males and 33 females) diagnosed Autistic patients, and 100 (55 males and 45 females) healthy controls without having any family history of Autism have been chosen from the Bengalee Hindu caste population of Kolkata, West Bengal, India. Bilateral Palm prints of every individual were collected according to the standard ink and roller method8 and classified according to Schaumann and Alter’s (1976) classification.5 The Papillary Number (PN) was computed by the sum of the termination point numbers of the four palmar main lines A, B, C & D.36,37 The Main Line Index (MLI), made up of the termination points of D and A lines, were added together and formulated as ‘(D+A)-5’.5 All the data were interpreted and analyzed in SPSS (version 16.0) for descriptive and inferential statistics. Cut-off values were set as p = 0.05 (Figure 1).
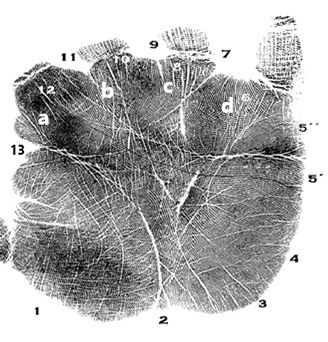
Figure 1 Pictorial presentation of Palmar Land Marks involves in MLF, MLI, C-line Termination and PN.
The present study received the Institutional Ethical Clearance from the ‘Institutional Ethical Committee for Bio-Medical and Health Research involving Human Participants, University of Calcutta’ vide Reference No. 06/ET/19-20/1742, dated 14.06.2019. When applicable, the parents and the Autism management center’s authority were given prior verbal and written consent. Anonymity was strictly followed for all the participants.
In the present study, Table 1 suggested that the significant (p<0.05) sexual dimorphism was found regarding PN within the Autistic patients while combining both hands, where the Autistic males posses higher value of PN (Table 1\) than that of the Autistic females. Nevertheless, Table 2 revealed that the significant (p<0.05) sexual dimorphism was found in the context of MLI within the Autistic patients while combining both hands, where the Autistic males possess higher value of MLI (Table 2) than that of the Autistic females. However, the controls never showed any sexual dimorphism regarding the PN (Table 1) and MLI (Table 2) while combining both hands.
|
PN |
Both hands |
Autistic males (n=134) |
Autistic females (n=66) |
|
28.13±3.17* |
26.53±3.90 |
||
|
Control males (n=110) |
Control females (n=90) |
||
|
27.66±4.15 |
27.26±3.74 |
Table 1 The distribution of PN among the Autistic males and Autistic females as well as Control males and Control females without any side differentiation
*p<0.05
|
PN |
Both hands |
Autistic males (n=134) |
Autistic females (n=66) |
|
8.71±1.95* |
7.88±1.88 |
||
|
Control males (n=110) |
Control females (n=90) |
||
|
8.57±2.37 |
8.14±2.14 |
Table 2 The distribution of MLI among the Autistic males and Autistic females as well as Control males and Control females without any side differentiation
*p<0.05
Unusual dermatoglyphic traits played a vital role in understanding the neurodevelopmental imbalances or the alteration of brain growths, which was well documented worldwide.2,3 As a complex neurodevelopmental imbalance1 the most extensive dermatoglyphic findings on Autism carried out in context of the lesser value of a-b ridge count and c-d ridge count,25,31–34 higher at d angle33 and presence of multiple axial tri radii in same palm.31,35 The present study incorporated the PN and MLI in the context of understanding sexual dimorphism. The dermatoglyphic traits were sheared the course of palmar main lines.37 PN’s outcome reflected the inclination of the four palmar main lines.37 Between those dermatoglyphic traits, MLI provides the result of the inclination of two palmar main lines, A and B, where the other two main lines were not taken in a count. However, the higher MLI demonstrated a horizontal course of the main lines (broad palmar area), and a lower index showed a vertical course (narrow palmar area).8,5 Apart from the several physiological traits, dermatoglyphics also poses sexual dimorphisms.8 Among the several dermatoglyphic traits, the PN and MLI also show sex differences.36,37,39 The present study also significant (p<0.05) sexual dimorphism was found in among the Autistic patients regarding the PN (Table 1) and MLI (Table 2), considering both the hands. The study also indicated that the Autistic males had significantly (p<0.05) higher value of PN (Table 1) as well as MLI (Table 2). It seems to be that Autistic males have a border inter-digital area along with more transverse palms than Autistic females. However, the controls never revealed the sexual dimorphism regarding the PN (Table 1) and MLI (Table 2). Therefore, the present study concluded that as Autistic patients show sexual dimorphism in connection with PN and MLI, therefore these might be used as another crucial dermatoglyphic trait for Autism prognosis.
The present study concluded as Autistic patients show sexual dimorphism in respect of PN and MLI, which may be used as another crucial dermatoglyphic trait for Autism prognosis. Moreover it can be useful as a cost-effective way to predict Autism just after the birth of a new born.
The authors are grateful to all the participants and the authority of different Autism management centers in West Bengal. We want to express our heartiest gratitude to the studied participant for their cooperation. Moreover, acknowledgment is also owed to the Department of Anthropology, U.C.S.T.A, University of Calcutta, for providing all the necessities for the present study.
None.
No conflict of interest exists.

©2023 Dey, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.