Journal of
eISSN: 2373-4426


Case Report Volume 5 Issue 7
Utrecht University, Netherlands
Correspondence: Yusuf Eken, Utrecht University, Beneluxlaan 753 1363BJ almere Holland, Netherlands, Tel 31647958978
Received: July 16, 2016 | Published: December 6, 2016
Citation: Eken Y (2016) Fever of Unknown Origin with Polyarthritis. J Pediatr Neonatal Care 5(7): 00214. DOI: 10.15406/jpnc.2016.05.00214
We describe a 14 years old male child presented with since 5 days of fever, polyarthritis and Salmon colored rash. For almost a century, this disorder is first recognized by George Frederic Still.1
Keywords: systemic onset JIA, juvenile idiopathic arthritis, salmon colored rash, polyarthritis, autoimmune, remitting fever
This disease has been defined as systemic arthritis by the International League of Associations for Rheumatology (ILAR) classification of juvenile idiopathic arthritis (JIA), 2 systemic-onset juvenile rheumatoid arthritis (JRA) by the American College of Rheumatology classification, or systemic-onset juvenile chronic arthritis by the European League against Rheumatism classification. Diagnosis of systemic arthritis by the ILAR criteria requires the presence of arthritis and a documented quotidian fever of at least 2 weeks’ duration, plus one of the following: typical rash, generalized lymphadenopathy, enlargement of liver or spleen, or serositis. Criteria and exclusions are shown in Table 1&2.2–9
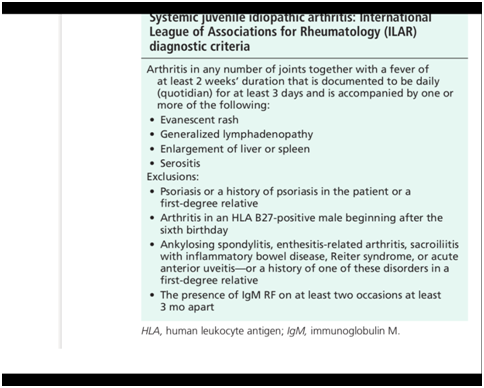
Table 1 Most common clinical features of systemic onset JIA.2
He complained of since 5 days pain in throat, skin rash and fever up to 39°C with since 3 days pain and swelling in the wrist and ankles. Physical Examination showed a child with normal vital signs. He was alert, good oriented in time/space and person. He had diffuse spread over his whole body salmon colored erythematous rash since 5 days (disappeared during admission after 6 days). ENT examination revealed red throat, Lymph nodules not enlarged. The lungs were clear to auscultation. There was no evidence of hepato-splenomegaly. Left knee painful at extension, not warmer than right knee, no swelling and painful wrists with limited movement
Laboratory tests (Table 3) showed striking elevation in indicators of inflammation. CRP reached a maximum value of 259 (in first week of admission), BSE 77. White cell count is elevated (Leukocytes total number) and with a predominance of polymorphonuclear leukocytes (neutrophils). Thrombocytes at admission 359, increased to near 600. Liver- and kidney function normal. LDH increased from 677 to 1018. ANA negative, ANCA negative. Reuma factors negative. Serologic tests for infection were negative for: Brucella, Bartonella, Leptospirose. EBV, Mycoplasma, Parvovirus B 19. Adenovirus, Coxsackie, Measles, CMV, Borrelia burgdorferi, Serologic test for a bacterial infection with streptococ bacteria showed elevation in antibody titer. (Table 3) Microbiological cultures remained negative. Fecal, blood, throat, urine cultures showed no recent infection. Fecal culture on Salmonella en Shigella, Campylobacter, Yersinia species was negative. Mantoux test for tuberculosis infection was negative.
Test |
Admission |
Week 1 |
Week 2 |
Week3 |
Leucocytes |
17.5 |
29.2 |
21 |
17.2 |
Neutrophils |
15.1 |
25.4 |
18.6 |
13.2 |
CRP |
164 |
259 |
217 |
45 |
BSE (mm/h) |
|
77 |
63 |
45 |
LD |
|
|
677 |
1018 |
Thrombocyts |
359 |
521 |
441 |
|
Table 3 Laboratory investigations
Note: Day 1: Antistreptolysine O titer, AST (aggl) 437, Anti DNAse B titer (aggl) 180
Day 35: AST 479 IE/ml, Anti DNA'se B 167 IE/ml
Radiological examination revealed no signs of malignancy. Roentgen image of thorax showed heart normal size, lungs normal, lymph nodules. Echo abdomen showed normal liver, kidney, spleen.
Skeletscintigrafie showed increased uptake at left foot and left wrist; meaning poly-arthritis / poly-synovitis. Roentgen image of left knee showed osteochondritis dissecans ECG: normal, no sign of pericarditis (no low voltage, no ST-segment elevation, no T-wave inversion) see Figure 1.
General discussion
This case study described a 14 years old child with persistent fever since two weeks, rash, and polyarthritis. The onset of the disease with features of recurrent fever and polyarthritis is nonspecific and may suggest bacterial or viral infection, malignancy, or another inflammatory disease. The most common clinical features in 136 children with systemic-onset JRA were fever (98%), arthritis (88%) and rash (81%). Only 39% had lymphadenopathy, 10% had pericarditis, and fewer had hepato-splenomegaly.10 Patient in our case had the required criteria for the diagnosis of systemic arthritis by the ILAR criteria. (Fever of at least 2 weeks’ duration, plus typical rash). Criteria and exclusions are shown in Table 1&2.2–9 The clinical features are similar to Adult onset Still's disease.3,4,8,9 When we compare our case of systemic onset JIA with Adult onset Still’s disease criteria, than we come to conclusion that the required criteria for Diagnosis of Still disease is similar. Child described in our case has 5 major criteria (fever >39 longer than 1 week, arthritis, salmon colored rash, leucocytosis > 10.000, with >80 % neutrophils) and one minor criteria (negative Rheumatoid Factor and ANA) according to Yamaguchi. Our case had 3 major criteria and one minor criteria according to Fautrel. See for ILAR criteria table 1, for criteria of Yamaguchi and Fautrel Table 2.2–8 The possibility of a childhood vasculitis or malignancy is excluded by radiological investigations and other clinical investigations. This exclusion criteria are also named in ILAR criteria and criteria for Still’s disease.
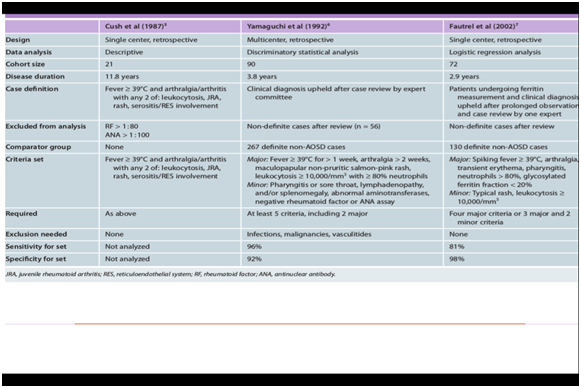
Table 2 Criteria for Still's disease5–7
This case shows that not the presence of the clinical evident features in the onset but the evolution of the disease eventually made the diagnosis of systemic onset juvenile idiopathic arthritis. About 40% of the children with systemic JIA follow a monocyclic disease course and eventually recover almost completely, after a variable period. A small proportion of children have a polycyclic course characterized by recurrent episodes of active disease interrupted by periods of remission without medications. Studies have shown that more than one-half of the children with systemic JIA have a persistent disease course which has resulted in progressive involvement of more and more joints and moderate to severe functional disability.11
The most important early predictors of destructive arthritis are polyarthritis, thrombocytosis, persistent fever, or the need for systemic corticosteroids in the first 6 months after disease onset.12,13 Treatment consisted in this case of NSAIDs. Patient received high dose Naproxen. Because of side effect is NSAID switched to Indometacine retard 75 mg. With NSAID is used both to aid in control of the systemic inflammatory features (e.g., fever) and to modulate joint pain and inflammation. Because systemic features seldom respond satisfactorily to NSAIDs alone, if the diagnosis is firmly established, the early use of glucocorticoids is indicated. Intravenous methylprednisolone (30 mg/kg/day to a maximum of 1 g/day on 1 to 3 consecutive days) is effective in controlling systemic and articular features of the disease, but the effect is often short-lived. Therefore, oral prednisone (1 to 2 mg/kg/day to a maximum of 60 mg/day in one or more doses) is often necessary. Disease-modifying antirheumatic drugs have been traditionally used in patients with s-JIA, with the goal of sparing glucocorticoids, but their efficacy is usually limited. Although most of the evidence is provided by uncontrolled studies, biologic agents that inhibit the three pivotal inflammatory cytokines (TNF, IL-1 and IL-6) have already changed the approach to the treatment of s-JIA.14–17 The role of especially IL-1 in the pathogenesis of s-JIA and predictors of response to IL-1 inhibition has been studied.16,18–21
Abnormal expression of three of the most important pro- inflammatory cytokines-interleukin-6 (lL-6), IL-l, and tumor necrosis factor-a (TNF-a)-is characteristic of systemic JIA. De Benedetti and Martini33 suggested that systemic JIA is an IL-6-mediated disease. Evidence to support that hypothesis is strong. IL-6 is markedly elevated in the blood and synovial fluid.22–24 The IL-6 level increases just before each fever spike and correlates with the systemic activity of the disease, arthritis, and increase in acute phase reactants.25–29 The abnormalities in regulation of IL-6 are also probably responsible for the limitation of growth, thrombocytosis. and microcytic anemia seen in this disease.27,28
There is accumulating evidence that inhibition of IL-1 or IL-6 is highly efficacious in a significant number of patients with persistent s-JIA, with improvements seen in both systemic symptoms and arthritis.30–34 The long-term benefits of these approaches still need to be determined. One of the complications of systemic onset juvenile arthritis is macrophage activation syndrome (MAS). MAS bears close resemblance to secondary hemophagocytic lymphohistiocytosis (HLH) and is associated with serious morbidity and sometimes death.35–38 Other complication of systemic onset JIA is secondary amyloidosis. The outcome of JIA-associated amyloidosis in the Finnish series was also poor, with a mortality rate of 42% and renal insufficiency or renal transplantation required in 25% of survivors, after a mean follow-up of 15 years.39 Autonomous stem cell transplantation for systemic onset JIA can be a future remedy. There have been studies in the past.40–49
Final diagnosis
Systemic onset juvenile idiopathic arthritis.
None.
The authors declare no conflict of interest.
None.

©2016 Eken. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Down Syndrome Day is observed on 21 March 2026 to raise awareness about Down syndrome and to
promote inclusion, early intervention, and quality healthcare for children with genetic conditions. This day highlights the importance
of pediatric monitoring, developmental support, and continued research to improve health outcomes and overall well-being.
Researchers, pediatricians, and healthcare professionals are invited to submit their original research articles, reviews, and clinical
studies related to Down syndrome, neonatal screening, developmental pediatrics, and child health. Manuscripts submitted on the occasion
of World Down Syndrome Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).
World Down Syndrome Day is observed on 21 March 2026 to raise awareness about Down syndrome and to
promote inclusion, early intervention, and quality healthcare for children with genetic conditions. This day highlights the importance
of pediatric monitoring, developmental support, and continued research to improve health outcomes and overall well-being.
Researchers, pediatricians, and healthcare professionals are invited to submit their original research articles, reviews, and clinical
studies related to Down syndrome, neonatal screening, developmental pediatrics, and child health. Manuscripts submitted on the occasion
of World Down Syndrome Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).