Journal of
eISSN: 2373-4426


Review Article Volume 6 Issue 6
1Consultant Neonatologist, Neonatal Intensive Care Unit, Pediatric Department, Latifa Women & Children Hospital, Dubai Health Authority, UAE
2Consultant Neonatologist and Head of Pediatric Department & Neonatal Intensive Care Unit, Latifa Women & Children Hospital, Dubai Health Authority, UAE
3Specialist Senior Registrar, Neonatal Intensive Care Unit, Pediatric Department, Latifa Women & Children Hospital, Dubai Health Authority, UAE
4Specialist Registrar, Neonatal Intensive Care Unit, Pediatric Department, Latifa Women & Children Hospital, Dubai Health Authority, UAE
Correspondence: Khaled El-Atawi, NICU, Pediatric Department, Latifa Women & Children Hospital, Dubai, UAE
Received: April 27, 2017 | Published: June 30, 2017
Citation: El-Atawi K, Elhalik M, Kulkarni T, Abdelsamed A, Alexander L, et al. (2017) Evolving Invasive Neonatal Systemic Candidiasis, a Review. J Pediatr Neonatal Care 6(6): 00271. DOI: 10.15406/jpnc.2017.06.00271
Preterm and low birth weight (LBW) infants are vulnerable to invasive candidiasis, usually caused by Candida albicans and Candida parapsilosis. Neonatal candidiasis, acquired after 6 days of life, is the most common form of invasive candidiasis in neonates. Once the Candida species penetrate any organ system, it can lead to complications such as meningitis, endocarditis, pyelonephritis, septic arthritis and pneumonia. In recent years, the incidence of invasive candidiasis has decreased in neonatal intensive care units (NICU) due to improvements in neonatal care; however, in the affected neonates, mortality remains a major concern. This is primarily attributable to the limitations of currently used diagnostic tests and delay in treatment. In addition, there is a lack of data on pharmacokinetics and pharmacodynamics of various anti-fungal therapeutic regimens in neonates. This review provides an overview of invasive candidiasis in neonates, including modes of transmission, its risk factors, and management, with special focus on the recent updates in diagnosis and treatment.
Keywords: anti-fungal, invasive candidiasis, neonatal candidiasis, neonates, systemic candidiasis, management
Preterm and low birth weight (LBW) infants are commonly susceptible to invasive candidiasis, usually caused by Candida albicans and Candida parapsilosis.1–5 Other non-albicans Candida species found to be associated with invasive candidiasis include: C. glabrata, C. tropicalis, C. lusitaniae, C. guilliermondii, C. pelliculosa, C. zeylanoides and C. krusei .1–4
Invasive candidiasis can either be acquired in-utero (within 6 days of life) or post-partum (after 6 days of life). If acquired in-utero, it is referred as congenital candidiasis (CC). CC is usually cutaneous initially and becomes systemic later, known as congenital systemic candidiasis. It is a rare form of invasive candidiasis with very few cases (around 100) reported in medical literature.6,7 The other type of infection is acquired after 6 days of life and is referred as neonatal candidiasis. It is the common form of invasive candidiasis in neonates.6
Once the Candida species enter blood stream, it is likely to penetrate in various body organs including central nervous system (CNS), heart, kidneys, liver, spleen and eyes. This can lead to complications such as meningitis, endocarditis, candida pyelonephritis, renal papillary necrosis, multiple parenchymal abscesses, endophthalmitis, septic arthritis and osteomyelitis, peritonitis, and pneumonia.8–10
With advances in neonatal management, incidence of invasive candidiasis has decreased in neonatal intensive care units (NICUs) over the years. A study by Aliaga et al. (2014) reported a decrease in the annual incidence of invasive candidiasis from 3.6 episodes per 1000 patients to 1.4 episodes per 1000 patients among all infants from 1997 to 2010. On the basis of birth weight, the incidence decreased from 24.2 to 11.6 episodes per 1000 patients among infants with a birth weight of 750-999 g, and from 82.7 to 23.8 episodes per 1000 patients among infants with a birth weight <750 g.11 Another study by Chitnis et al. (2010) assessed the incidence of Candida species central line-associated bloodstream infections (CLABSIs) in US NICUs from 1999 to 2009. The study results showed a significant decrease in the incidence of CLABSIs due to Candida species (pooled mean rate of Candida spp. CLABSIs per 1000 central line-days reduced from 0.9 in 1999 to 0.2 in 2009; p < 0.001).12 However, the mortality rate associated with invasive candidiasis is reported to range from 19.6% to 63% and remains a major concern.1,4,13
Despite the decrease in incidence of invasive candidiasis in neonates, the associated mortality rate is high in preterm and very LBW infants. High mortality can be attributed to the limitations of routinely used diagnostic tests (such as longer duration and insufficient amount of sample for testing) and delay in treatment (due to longer time taken for confirmation of diagnosis and lack of sufficient data on dosing of antifungal therapy in neonates).8,10,14 Although, various antifungals have been developed for treatment of invasive candidiasis, there is scarcity of data on their pharmacodynamics and pharmacokinetics in neonates and children.15 All these factors highlight the need for appropriate diagnosis and management of neonatal candidiasis.
The present review discusses the overview of invasive candidiasis in neonates, modes of transmission, its risk factors, and neurodevelopmental outcomes. Further, the review explains the diagnosis and treatment of invasive candidiasis in neonates, with special focus on the recent updates in its management.
Candidalinfections can be transmitted in neonates via two modes:
Risk factors
Multiple risk factors in neonates contribute to invasive candidiasis. LBW (>2500 g) and earlier gestational age (29–32 weeks) are found to be commonly associated with invasive candidiasis in neonates. Other risk factors identified in clinical studies to be associated with invasive candidiasis are vaginal birth, central catheters, day of life (>7), use of broad-spectrum antibiotics in neonates, antenatal steroids, premature rupture of membranes, mechanical ventilation, necrotizing enterocolitis and parenteral nutrition (Table 1).1,14,13,17
|
Patient population |
Risk factors |
|
|
Barton et al.13 |
N (cases) = 49 |
· NEC (OR: 4.81 .95% CI: 1.14-20.41; p = 0.03) |
|
N (control) = 90 |
||
|
Lee et al..51 |
N = 330 (> 1500 g) |
· Day of life >7 (OR 25.2; 95% CI 14.6–43.3) |
|
· Vaginal birth (OR 1.6 .1.2–2.3) |
||
|
· Central venous line (OR 1.8 .1.3–2.6) |
||
|
· Exposure to broad-spectrum antibiotics (OR 1.6 .1.1–2.4) |
||
|
· Platelet count <50,000/mm3 (OR 3.7 .2.1–6.7) |
||
|
Oeser et al.52 |
N = 98 |
· NEC: 24 (30%) |
|
· Respiratory support: 73 (92%) |
||
|
· CVC: 51 (61%) |
||
|
· PN within 48 h: 77 (94%) |
||
|
· Trophic feeds within 48 h: 52 (63%) |
||
|
· Receipt of systemic corticosteroids: 8 (10%) |
||
|
· H2 receptor antagonists: 10 (12%) |
||
|
· Insulin 16 (20%) |
||
|
· Previous abdominal surgery: 11 (13%) |
||
|
Wadile et al.53 |
N = 108 |
· Intrapartum use of broad-spectrum antibiotics: 59 (95.16%) |
|
· Prematurity: 38 (61.29%) |
||
|
· LBW: 47 (79.03%) |
||
|
· Indwelling catheters: 5 (8.06%) |
||
|
· Artificial ventilation: 26 (41.94%) |
||
|
· Male sex: 37 (59.67%) |
||
|
· Vaginal delivery: 53 (85.48%) |
||
|
Khan et al.17 |
N= 560 (36%) had positive cultures. Candida was isolated in 49 (8.8%) neonates with positive cultures. |
· Mechanical ventilation: 41 (83.7%) |
|
· Prior antibiotic therapy: 45 (92%) |
||
|
· Partial PN 15 (30%) |
Table 1 Studies Assessing Risk Factors for Invasive Candidiasis in Neonates.
*NEC, necrotizing enterocolitis; PN, parenteral nutrition
Neurodevelopment outcomes in neonates with invasive candidiasis
Invasive candidiasis in neonatal survivors is found to be associated with adverse neurodevelopmental outcomes. A study by Benjamin et al. (2006) reported neurodevelopmental impairment (NDI) or mortality in 73% of infants with invasive candidiasis. Moreover, infants who had delayed removal or replacement of central catheters (>1 day after initiation of antifungal therapy) were at increased risk of NDI.18
In a study by De Hann et al.(2013), 29 neonates who survived Candida sepsis were followed up at 24 months to assess the neurological outcomes. Of the 29 neonates, 17 appeared for follow-up, and neurological examination showed mild and severe abnormality in 9 (53%) and 1 (6%) infants, respectively. The median value of Bayley Psychomotor Development Index (PDI) and Bayley Mental Development Index (MDI) were 76 (59-100) and 92 (78-108), respectively. Hearing disabilities and visual impairment occurred in 4 (24%) and 3 (18%) infants, respectively.19 Another study by Adams-Chapman et al. (2013) assessed the neurodevelopmental outcomes of extremely LBW infants who survived following sepsis with Candida infection or non-Candida sepsis at 18 months of age. The study reported that 31% of infants in each group (with Candida sepsis and non-Candida sepsis) had NDI at 18 months. However, infants with Candida sepsis (OR: 1.83; 95% CI: =1.83 [1.01-3.33], p= 0.047) were reported to be at increased risk of NDI as compared to uninfected infants.20
Another study assessed the neurodevelopmental outcomes in infants (≤1000 g) with invasive candidiasis who were receiving empirical antifungal therapy. The study reported that incidence of death or NDI was less in infants who received empirical antifungal therapy (19/38, 50%) compared with those who had not (55/86, 64%; OR = 0.27 [95% CI 0.08–0.86]. It was suggested that empirical antifungal prophylaxis should be initiated in LBW infants with risk of fungal infections to improve neurodevelopmental outcomes.2
Diagnosis
Rapid diagnosis is the best possible way to successfully manage the preterm or full-term neonate with invasive candidiasis.
Body fluids culture: Blood culture is the most common diagnostic procedure used for detection of candidiasis.14 Other commonly used methods include culture/microscopic examination of urine, cerebrospinal fluid, peritoneal fluid and other sterile body fluids.10
However, blood culture to confirm diagnosis of invasive candidiasis in neonates has several limitations. Firstly, the availability of less amount of blood (0.5 to 1 mL) for culturing makes it difficult to isolate Candida in neonates. Secondly, the median time taken by blood culture to detect Candida species in infants is 36 hrs and might reach 42 hrs, if the infant is receiving antifungal therapy prior to blood culturing.14 Further, speciation and susceptibility testing following isolation from blood culture adds on more days for diagnosis.21
Also, the negative cerebrospinal fluid (CSF) culture in infants does not exclude the presence of candida meningitis. A study reported presence of normal CSF parameters in almost half of the study patients with Candida meningitis.22 Thus, new diagnostic methods are being investigated to overcome the limitations associated with the culturing method.
Peptide nucleic acid fluorescence in situ hybridization (PNA FISH): Once the blood culture turns out to be positive, technique like PNA FISH can be used to shorten the time required for speciation.21 A recent study compared the compatibility of the peptide nucleic acid fluorescent in situ hybridization Yeast Traffic Light (PNA FISH® YTL) with VITEK 2 System in early identification of Candida spp. directly from positive blood cultures (n = 15) and other clinically significant specimens (n = 10). The study reported assay identification compatibility of PNA FISH® YTL with VITEK 2 System in 21/25 (84%) specimens tested, and suggested that this assay can be used to identify Candida from blood cultures, urine cultures, peritoneal fluid cultures and catheter tip cultures.23 Although this test appears to be clinically beneficial, its use is limited due to several factors such as lack of specificity, requirement of a positive blood culture, and inability to distinguish between all clinically relevant Candida species.21
Polymerase chain reaction: Is being studied to identify Candida infection in neonates more rapidly and with higher sensitivity. PCR also makes it feasible to use small amount of blood to detect Candida infection.10,24,25 Table 2 presents studies evaluating the use of PCR in neonates with invasive candidiasis.
|
Studies |
Type of Study |
Patient population |
Diagnostic technique assessed |
Outcomes |
|
Zhao et al. 29 |
Case control study |
63 preterm infants with IFD, 160 preterm infants without sepsis (preterm control), and 41 preterm infants with bacterial sepsis |
BG and platelet parameters (PC, PCT, PDW and MPV) |
Sensitivity and specificity of BG: 68.3% and 75.6%; PC: 78% and 95%, and PCT: 83% and 85%, respectively |
|
Taira et al.25 |
Prospective study |
54 pediatric patients (24 neonates) |
BC and PCR |
BC and multiplex nested PCR were positive in 14.8% vs 24.0% of patients, respectively. |
|
Goudjil et al.54 |
Retrospective study |
Infected (n = 18) or non-infected (n = 43) neonates |
BG l |
BG level was significantly higher in the infected group (364 pg/mL .13-1976) as compared to non-infected group (89 pg/mL .30–127; p < 0.001). |
|
Oliveri et al.55 |
Observational study |
N= 70 pre-term infants |
Mannan antigen test |
Overall sensitivity and specificity of the assay was 94.4% and 94.2%, respectively and culture-proven sensitivity and specificity was 92% and 84%, respectively |
|
Mularoni et al. |
Case series |
N = 4 (2 low-birth-weight neonates and 2 stem cell transplant recipients) |
BG test |
Plasmatic levels of BG were >523 pg/mL. BG test might be used to identify IFD in pediatrics |
Table 2 Studies Assessing the Use of Molecular Diagnostic Tests in Neonatal Candidiasis.
*IFD, invasive fungal disease; BG, (1, 3)-β-D-glucan; PC: Platelet count, PCT plateletcrit; PDW, platelet distribution width; MPV, mean platelet volume; BC, blood culture
A meta-analysis of 23 studies evaluating efficacy of PCR and other hybridization methods in diagnosis of neonatal sepsis concluded that molecular techniques cannot replace culture methods presently due to insufficient sensitivity, although they can be used as an add-on to culture methods.26 A review by Paolucci et al. highlighted that lack of sensitivity could be attributed to varied amount of blood collected in different studies (200 µL to 2 mL).27 Moreover, there is lack of studies on standardization and validation of PCR in neonates.28 PCR use is further limited due to likelihood for sample contamination (leading to false-positive results), lack of probes with capability to detect multiple Candida species, and difficulty in sample preparation.21 Thus, more studies need to be conducted to assess the validity and reliability of PCR and other molecular techniques in neonates.
Biomarkers: Researchers are also investigating the use of markers of fungal disease in diagnosis such as (1, 3)-β-D-Glucan (present in fungal cell wall), D-arabinitol, anti-Candida antibodies, mannan antigen, and fungal chitin synthase.10,29 Very few studies have been conducted in neonates on usability of these biomarkers in diagnosis of invasive candidiasis (Table 2). Although these tests show encouraging results, cost and lack of substantial data in neonates prevent their use in standard practice presently.
Other diagnostic tests: Neonates with persistent candidemia are likely to have end-organ dissemination (EOD). Thus, it is suggested to screen EOD using renal and cranial ultrasound/ magnetic resonance imaging (MRI), echocardiogram and ophthalmologic examination, either prior to initiation of the therapy or after 5-7 days of the initiation of the treatment. Abdominal ultrasound is suggested to assess for peritoneal, splenic or liver involvement.10
There is lack of data on efficacy and safety of antifungals in neonates. The studies conducted till date have only focused on pharmacokinetics and pharmacodynamics evaluations of the antifungal agents. However, the recent updated guidelines from the Infectious Diseases Society of America (IDSA) (2016) presented recommendations for management of invasive candidiasis in neonates based on the available clinical data. The European Society of Clinical Microbiology and Infectious Disease (ESCMID) also released guidelines on the diagnosis and management of invasive candidiasis in neonates.30
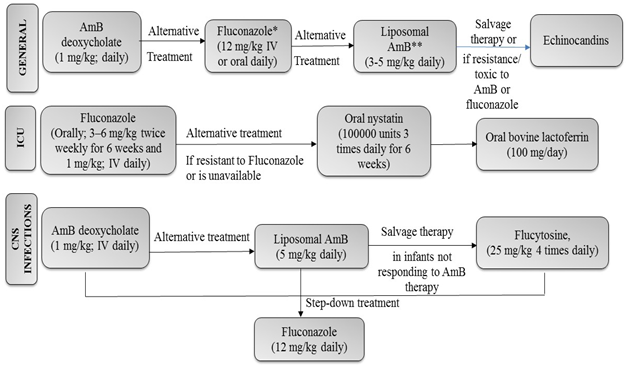
Figure 1 Summary of Recommendations on Management of Invasive Candidiasis in Neonates as per IDSA Guidelines. IV: intravenous; CSF, cerebrospinal fluid; CNS, central nervous system; ICU,Intensive care unit, Amp B, amphotericin B. *In patients who have not been on fluconazole prophylaxis **To be used cautiously, particularly in the presence of urinary tract involvement.50
The lipid-based formulations i.e. amphotericin B liposomal complex (ABLC) are also being used to treat invasive candidiasis in neonates. ABLC has received a grading of B-III and B-II in ISDA and ESCMID guidelines, respectively, for use in neonates with invasive candidiasis.30,31 Although the safety of liposomal formulations of Amp B has only been assessed in some observational uncontrolled studies, results have been favorable.33,34 However, there is lack of recent data evaluating the safety of ABLC in neonates. Moreover, there are few studies comparing ABLC with other therapies including Amp B. A recent study compared the use of ABLC with conventional Amp B in infants (≤120 days of age) who had confirmed diagnosis of invasive candidiasis. The study reported increased mortality rate and treatment failure for neonates receiving lipid formulations compared with Amp (OR: 1.96 [95% CI: 1.16, 3.33]; p = 0.01.35 Further well-designed studies (randomized) need to be conducted to assess the efficacy and safety of ABLC in neonates.
On the other hand, a study by Ascher et al. reported the use of D-Amp B, fluconazole, Amp B lipid products, or combination therapy in 730 infants. Multivariable regression showed no difference in the duration of candidiasis for infants treated with D-Amp B, Amp B lipid products, fluconazole, or combination therapy (p = 0.47). There was no significant difference in therapy failure rates for fluconazole and combination therapy compared with D-Amp B.35 No recommendation has been made in guidelines on the use of combination therapy in neonates.31
Other antifungals: Use of some other antifungals including Itraconazole, Voriconazole and anidulafungin have been assessed in very few studies in neonates, mainly limited to case reports. Thus, their use is not recommended in clinical practice.46 Several new antifungals such as Posaconazole, Ravuconazole, 5-Flucytosine, Isavuconazole and Posaconazole have also not been studied in neonates so far.38,46
Probiotics: Several studies are being conducted to assess the use of probiotics in treatment of late onset sepsis (LOS) in neonates. A recent meta-analysis by Rao et al. systematically reviewed 37 randomized clinical trials comparing probiotics with placebo and control in preterm infants with LOS. Probiotics significantly decreased the risk of LOS (675/4852 [13.9%] vs 744/4564 [16.3%]; relative risk [RR]: 0.86; 95% CI: 0.78–0.94; p = 0.0007).47 A meta-analysis by Zhang et al. (2016) also reported significant reduction in fungal sepsis (RR 0.57, 95% CI: 0.41–0.78) with enteral probiotic supplementation in preterm neonates with LOS.48 Although several studies have shown that probiotics play an important role in preventing Candida colonization in neonates with invasive candidiasis (IC), more multicenter clinical trials are needed to evaluate efficacy, safety, dosage, duration and strains (type and single or combination of different strains) of probiotics in prevention of Candida colonization and invasive candidiasis.49 Lactoferrin alone or in combination with Lactobacillus is suggested to be equally useful by the ESCMID guidelines.30. However, no recommendation on their use is given in the recent updated guidelines by ISDA.
An expert opinion states that researchers need to conduct studies assessing the efficacy and safety of antifungals in neonatal population because data from adult trials cannot be implied to neonates with invasive candidiasis due to differences in pathophysiology of the disease in neonates and adults.46
Neonates with LBW (<2500 g) and early gestational age (29–32 weeks) are at increased risk of developing invasive candidiasis. Other risk factors include vaginal birth, central catheters, day of life (>7), use of broad spectrum antibiotics, antenatal steroids, premature rupture of membranes, mechanical ventilation, necrotizing enterocolitis and parenteral nutrition. High mortality rate associated with invasive candidiasis in neonates necessitates the need to fill the gaps in its diagnosis and treatment. Also, adverse neurodevelopmental outcomes have been reported in neonates with invasive candidiasis. Use of empiric anti-fungal therapy is suggested to help in improving neurodevelopmental outcomes in neonates with invasive candidiasis. Culturing of blood and other body fluids is the conventional method used for diagnosis of invasive candidiasis. However, it leads to delay in diagnosis due to time-consuming procedure. Other molecular techniques including PCR and biomarkers are being investigated to be used for rapid diagnosis; however, no recommendations have been made in international guidelines. Also, there is lack of substantial data on efficacy and safety of antifungals in infants with invasive candidiasis. However, recommendations have been made in guidelines based on the available data. The guidelines have strongly recommended the use of D-Amp B and flucanozole in neonatal candidiasis. Other therapies including liposomal formulations of Amp B, oral nystatin, echinocandins, flucytosine and Lactoferrin are suggested as alternatives. In future, there is a need to conduct more studies in large population size and assess the long-term outcomes following antifungal prophylaxis in neonatal invasive candidiasis.
None.
The authors declare no conflicts of interest.

©2017 El-Atawi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 International Childhood Cancer Day is observed on 15 February 2026 to raise awareness about childhood cancers and to highlight the medical, developmental, and supportive care needs of affected children and their families. This day emphasizes the importance of early diagnosis, pediatric care, and continued research to improve survival and quality of life in children with cancer.
Researchers and healthcare professionals are encouraged to submit their original research articles, reviews, and clinical studies related to pediatric oncology, neonatal care, and child health. Manuscripts submitted on the occasion of International Childhood Cancer Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).
.
International Childhood Cancer Day is observed on 15 February 2026 to raise awareness about childhood cancers and to highlight the medical, developmental, and supportive care needs of affected children and their families. This day emphasizes the importance of early diagnosis, pediatric care, and continued research to improve survival and quality of life in children with cancer.
Researchers and healthcare professionals are encouraged to submit their original research articles, reviews, and clinical studies related to pediatric oncology, neonatal care, and child health. Manuscripts submitted on the occasion of International Childhood Cancer Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).
.