Journal of
eISSN: 2373-4426


Research Article Volume 14 Issue 1
1Pediatrics, CAP Alhambra, L'Hospitalet de Llobregat, Spain
2Pediatrics, Àrea Bàsica de Salut Montclar, Sant Boi de Llobregat, Spain
3Pranarȏm International S.A. 37, Avenue des artisans, 7822 Ghislenghien, Belgium
4Pediatrics, Centro Médico Adeslas, Leon, Spain
5Family Medicine, Centro de Atención Primaria (CAP), Corbera de Llobregat, Spain
6Pediatrics, Hosptial HM Nens, Spain
7Plant Biotechnology Research Unit, Université Libre de Bruxelles (ULB), Belgium
Correspondence: Eva Pacheco, Pediatrics, CAP Alhambra, L'Hospitalet de Llobregat, Barcelona, Spain, Tel 935177609
Received: December 21, 2023 | Published: January 17, 2024
Citation: Pacheco E, Crespo C, Mascret A, et al. Effectiveness and safety of AROMAFORCE® junior cough syrup in pediatric patients with acute upper respiratory conditions. J Pediatr Neonatal Care. 2024;14(1):14-20. DOI: 10.15406/jpnc.2024.14.00535
Aim: The purpose of this study was to evaluate the effectiveness and safety of Aromaforce® Junior Cough Syrup (AJCS) in treating acute upper respiratory tract infection-related coughs in children, and it served as a post-marketing clinical follow-up.
Methods: Prospective, multicenter, open-label, controlled clinical investigation conducted under normal conditions of use to evaluate the antitussive effectiveness of a mucilage-based syrup in pediatric patients (aged 2 to 12 years) as compared to increased hydration measures (control group), with a 1-week follow-up. Likert severity scores were used to evaluate coughs and related symptom severity.
Results: The results demonstrate that AJCS effectively reduces cough severity, including daytime and night-time cough, and the number of times the child was woken up, particularly within the first three days, surpassing the effectiveness of hydration measures. The results of the degree of satisfaction with AJCS show that the majority of physicians and parents had positive feedback. Furthermore, the safety analysis confirms the syrup's non-toxic nature in children. However, the initial differences in baseline characteristics between the study and control groups, with the study group exhibiting higher combined cough scores, limit the strength of the evidence.
Conclusions: This study provides further evidence supporting the efficacy and safety of AJCS in the treatment of cough associated with acute upper respiratory tract infections in children. Further randomized studies may provide further evidence of the efficacy and safety of AJCS.
Keywords: syrup, cough, upper respiratory tract infection, protective barrier, essential oils
Cough, characterized as a forceful expiration against a closed glottis that produces a distinct sound, acts to shield the airways by blocking food and liquids and removing excess substances.1,2 Cough is often considered the most troublesome symptom experienced by children and their parents during upper respiratory tract infections and is a common reason for pediatrician visits due to the discomfort it causes and its impact on sleep quality for both children and their families.3,4 This, in turn, leads to school and work absenteeism and increased healthcare costs.5,6
When treating cough symptoms, it is important to find a balance between modulating the cough reflex and preserving its physiological defense function. The treatment should focus on reducing inflammation and forming a protective barrier to prevent contact with irritating external agents, thus decreasing respiratory tract irritation.1 Up to 85% of the efficacy of cough syrups can be attributed to the syrup's physical and chemical properties, which provide a soothing or demulcent effect.7 Various types of symptomatic cough therapies are available, including mucolytic, expectorants, antitussives, demulcents, direct bronchodilators, and anti-inflammatory glucocorticoids.8 While these treatments are effective, many antitussives carry potential adverse effects, especially for the pediatric population.8 In recent years, there has been a growing interest in the use of natural substances for cough treatment due to their lower incidence of adverse effects.8–11 Many plants have been traditionally used in cough remedies, with some possessing calming properties (forming a protective barrier), expectorant properties (aiding mucus clearance), antispasmodic properties (relieving bronchial spasm), or antiseptic properties.12–14 Even natural products like honey have been the subject of recent studies in the field of cough treatment.15,16 The World Health Organization has recognized honey as a potential demulcent treatment for cough.17 Demulcents are mucilaginous substances that create a soothing protective layer over mucous membranes, alleviating mild pain and inflammation of the membrane.18 They stimulate saliva production and help suppress the cough reflex.18 Demulcents are highly valuable due to their rich mucilage content. Plant mucilage is a gel-like substance primarily composed of polysaccharides, uronic acid, glycoproteins, and other biologically-active compounds such as tannins, alkaloids, and steroids.14 Mucilages have been utilized for their antioxidant, antidiabetic, anticancer, antifungal, antimicrobial, anti-inflammatory, wound healing, ACE inhibiting, hypolipidemic, and immune-stimulating properties in several disorders.19 Mucilage products are also noteworthy for their properties of mucosal bioadhesion and protection against harmful irritants, which appear to be responsible for the antitussive activity of these compounds.7
Aromaforce® Junior Cough Syrup (AJCS) is a mucilage-based cough syrup, designed for pediatric patients with acute upper respiratory conditions. AJCS incorporates xanthan gum and gellan gum, two mucilages used widely in the food and pharmaceutical industry. These mucilages are non-absorbable, non-irritating, non-sensitizing, and non-toxic, making them suitable for various formulations. AJCS also incorporates essential oils, such as Citrus limon fruit, Lavandula angustifolia, Pinus sylvestris, Thymus vulgaris QT Thujanol, and Cinnamomum cassia. These oils have a history of traditional use as cough remedies and have been the subject of various studies that have highlighted their antibacterial, antioxidative, and anti-inflammatory properties.20–24
This clinical investigation aims to assess the effectiveness and safety of AJCS in treating coughs associated with acute upper respiratory tract infections in children. This study serves as a post-marketing clinical follow-up to further understand the performance of AJCS.
Study design and participants
This study is a prospective, multicenter, open-label, controlled clinical investigation with a CE-marked medical device under normal conditions of use to evaluate the antitussive effectiveness of a mucilage-based syrup in pediatric patients as compared to no treatment for cough and the recommendation to increase hydration (control group), with 1-week of follow-up.
The inclusion criteria for the study were children aged 2 to 12 years with a body weight of ≥11 kg, who were seen at the clinic for coughs secondary to acute upper respiratory tract infections, and whose parents agreed to their participation in the clinical investigation and signed the informed consent on behalf of the child they legally represent.
The exclusion criteria included children with a history of bronchial asthma, bronchitis, chronic respiratory disease, seizures, epilepsy, or any type of clinical disorder that, in the physician's opinion, could endanger the patient or interfere with the results of the clinical investigation. Other exclusion criteria were patients requiring antibiotic treatment, those using another treatment for cough, those with a known intolerance or allergy to any component of the syrup, and those with a low expectation of compliance with the clinical research plan. Patients for whom the physician requested antigen or PCR tests due to suspected SARS-CoV-2 infection or who had a positive SARS-CoV-2 antigen test in the last 7 days were also excluded from the study.
All patients who met the inclusion and exclusion criteria were enrolled in the study during the period from March 15th to December 12th, 2022.
The patients were divided into two groups based on the type of treatment they received. In the control group, patients were advised to maintain adequate hydration by consuming plenty of fluids throughout the day, following standard practice guidelines. On the other hand, patients in the study group were administered the investigational device (AJCS), starting from the day they were included at the clinic, and adhered to the recommended usage instructions. For children weighing between 11 kg and less than 20 kg (2-6 years), the treatment involved taking 1 stick twice a day, while children weighing between 20 kg and up to 40 kg (6-12 years) received 1 stick three times a day.
Patients were monitored for a period of one week, during which they had scheduled appointments with the pediatrician at the beginning of the study (baseline) and on Day 7 (end of study visit). Additionally, a telephone visit was conducted on Day 3 to assess the progress of the subjects. Moreover, parents completed a patient diary providing information on cough status every day during the follow-up week of the study.
All patients signed a written informed consent at the time of enrollment. The study protocol was approved by the independent ethics committee, CEIm IDIAP Jordi Gol i Gurina, at its meeting on January 26th, 2022. This research adhered to the principles outlined in the Declaration of Helsinki and complied with the regulations set forth in the EU General Data Protection Regulation (GDPR). In accordance with GDPR guidelines, all personal data was properly anonymized and securely stored separately from the research results.
Objectives and variables
The primary endpoint of interest was the measure of decrease in cough from the baseline visit to Day 3, assessed as the global score obtained in the Likert cough assessment severity questionnaire based on 6-point Likert scales (0=not at all, 1=not very much, 2=a little, 3=somewhat, 4=quite a bit, 5=a lot, 6=very much/extremely).25
The secondary outcomes measured the changes in night-time and daytime cough severity, discomfort caused by cough, child's ability to fall asleep and stay asleep, and parents/caregivers' ability to fall asleep and stay asleep. These assessments were conducted between the baseline visit and the visits on Day 3 and Day 7, using Likert scales ranging from 0 to 6.
Adverse events (AEs) were monitored throughout the clinical study. AEs were described as undesirable medical events that were or were not associated with the procedures or the product.
Finally, parent and physician satisfaction with the syrup was also measured through a 5-point Likert scale (0=very dissatisfied; 1=dissatisfied; 2=indifferent; 3=satisfied; 4=very satisfied).
Statistical analysis
Continuous variables were presented using measures of central tendency (mean) and dispersion (standard deviation), while categorical or ordinal variables were described using frequencies (n) and percentages (%). For the analysis of primary and secondary efficacy variables, comparisons were conducted using the Wilcoxon signed-rank test and Mann-Whitney U test. The safety analysis was descriptive in nature, and no statistical tests were performed.
A two-sided alpha (α) threshold of less than 0.05 (p<0.05) was considered statistically significant for all analyses. The data collected were analyzed using SAS (Statistical Analysis System) software, version 9.1.3 Service Pack 4 (SAS Institute, Inc., Cary, North Carolina, USA)
Demographic and clinical characteristics of study patients
Out of the 50 pediatric patients initially recruited, one patient in the study group was excluded for not meeting the inclusion criterion. This resulted in a valid population of 49 patients, with 29 in the study group and 20 in the control group. Two patients were withdrawn from the study due to respiratory complications, but their efficacy data was recorded up to Day 3.
A total of 22 males (44.9%) and 27 females (55.1%) were included in the study, with a mean (SD) age of 6.4 (2.9) years (range: 2 – 12 years). There were no significant differences observed between the groups in terms of sex distribution and age (Table 1).
|
|
Control group (n=20) |
Study group (n=29) |
|
Sex, n (%) |
||
|
Female |
11 (55.00%) |
16 (55.20%) |
|
Male |
9 (45.00%) |
13 (44.80%) |
|
Age, Mean (SD) |
6.27 (2.96) |
6.44 (3.05) |
Table 1 Demographic characteristics of the patients according to the study group
Baseline assessment of cough
Before starting the study, patients reported an average duration of 3.9 ± 4.1 days with cough, and no significant differences were observed between the two groups (Table 2).
|
|
Control group (n=20) |
Study group (n=29) |
p-value |
|
Days with cough*, n (%) |
2.94 (1,924) |
4.69 (5,089) |
n.s. |
|
Combined Cough Scores at Baseline |
|||
|
Daytime cough combined score (range: 0-18) |
8.40 (2.62) |
10.62 (3.28) |
<0.05 |
|
Night cough combined score (range: 0-30) |
11.50 (8.74) |
16.86 (8.30) |
<0.05 |
|
Overall Combined Score (range: 0-48) |
19.90 (10.73) |
27.48 (10.29) |
<0.05 |
|
Daytime cough |
|||
|
How frequent? |
2.90 (0.97) |
3.66 (1.14) |
<0.05 |
|
How severe? |
2.90 (1.12) |
3.62 (1.12) |
<0.05 |
|
How bothersome? |
2.60 (1.27) |
3.34 (1.42) |
n.s. |
|
Night-time cough |
6.27 (2.96) |
6.44 (3.05) |
|
|
How frequent? |
2.55 (1.82) |
3.55 (1.78) |
n.s. |
|
How severe? |
2.35 (1.66) |
3.62 (1.72) |
<0.05 |
|
How bothersome? |
2.35 (1.81) |
3.41 (1.74) |
n.s. |
|
How much did cough affect your child's ability to sleep? |
2.05 (1.76) |
3.25 (1.80) |
<0.05 |
|
How much did cough affect your (parents') ability to sleep? |
2.20 (1.99) |
3.14 (2.00) |
n.s. |
|
Number of times the child wakes up at night |
1.7 (1.8) |
2.7 (2.1) |
n.s. |
Table 2 Baseline assessment of cough
At baseline, the study group showed significantly higher scores for the daytime cough combined score, night-time cough combined score, and overall combined score compared to the control group. Regarding daytime cough, the study group reported a significantly higher frequency and severity of cough compared to the control group. For nocturnal cough, the study group experienced significantly more severe coughing and greater interference with the child's ability to sleep compared to the control group. However, no significant differences were observed in terms of cough severity, discomfort, and impact on the parents' ability to sleep. The mean number of times the child was woken up at night due to coughing at baseline was 2.24 ± 2.0 times (range: 0 to 10), and there were no significant differences between the groups (1.7 ± 1.8 vs. 2.7 ± 2.1; p=n.s.).
Other symptoms accompanying cough included nasal congestion (55.1%), rhinorrhea (49.0%), expectoration (34.7%), and sneezing (32.7%), with no significant differences observed between the groups.
Efficacy
During the Day 3 telephone follow-up, it was observed that 94.7% of the subjects in the control group and 96.6% of the subjects in the study group still had cough symptoms. Significant improvement in the combined score for daytime cough was observed in both groups (Figure 1). However, improvement in night-time cough and the overall combined score was significant only in the study group on Day 3. Additionally, the study group showed a greater reduction in all combined cough scores compared to the control group.
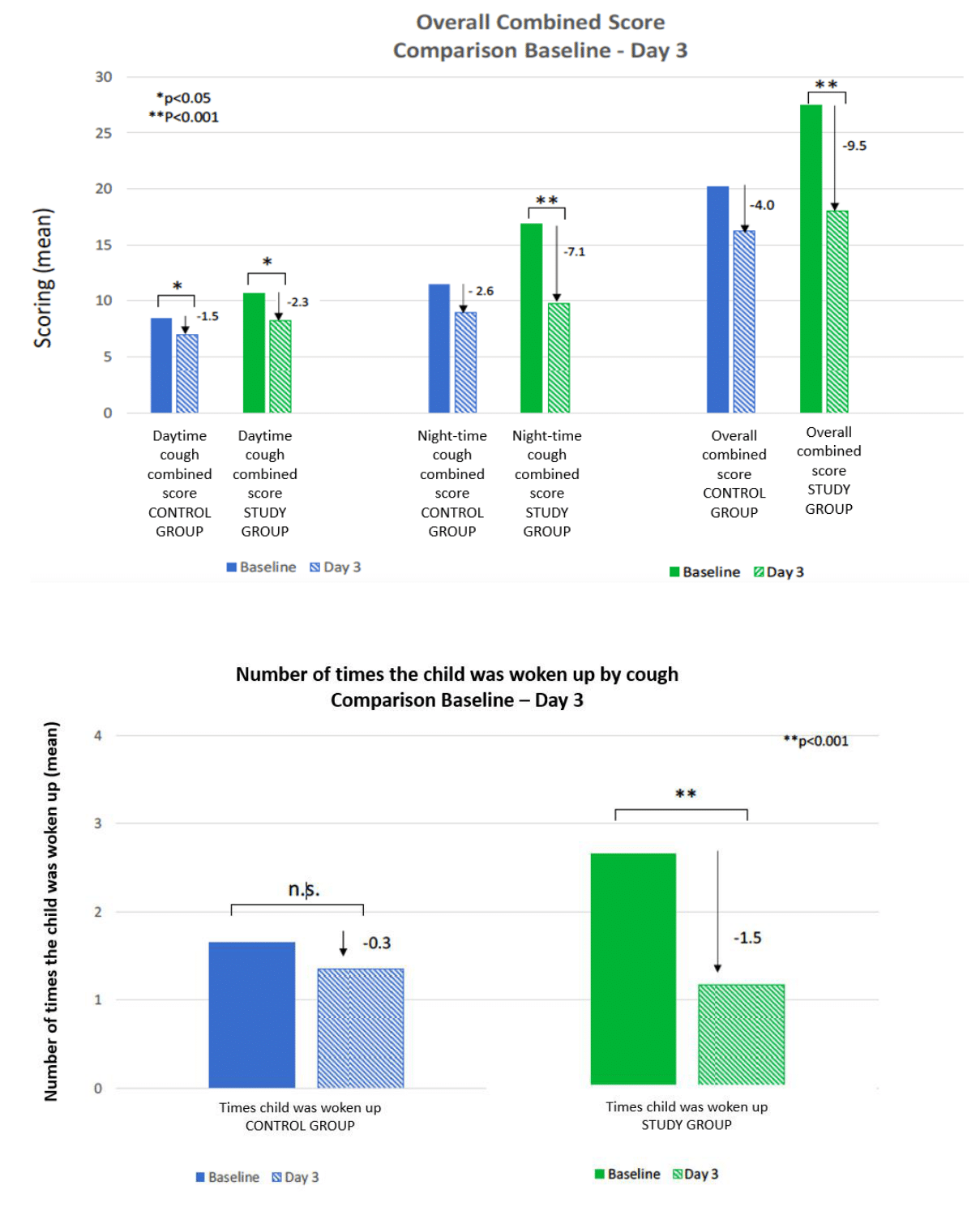
Figure 1 Comparison baselines -Day 3.
A) Overall Combined Score.
B) Number of times the child was woken up by cough.
The study group experienced a greater decrease in the number of times patients woke up coughing on Day 3 compared to the control group. This improvement was significant only in the study group, while no significant improvement was observed in the control group.
By the end of Day 7, 41.2% of the subjects in the control group and 48.3% of the subjects in the study group still had a cough without significant difference between the two groups. Both the control and study groups exhibited a significant decrease in all combined scores from baseline to Day 7 (Figure 2). However, the study group demonstrated significantly higher decreases in all combined scores compared to the control group.
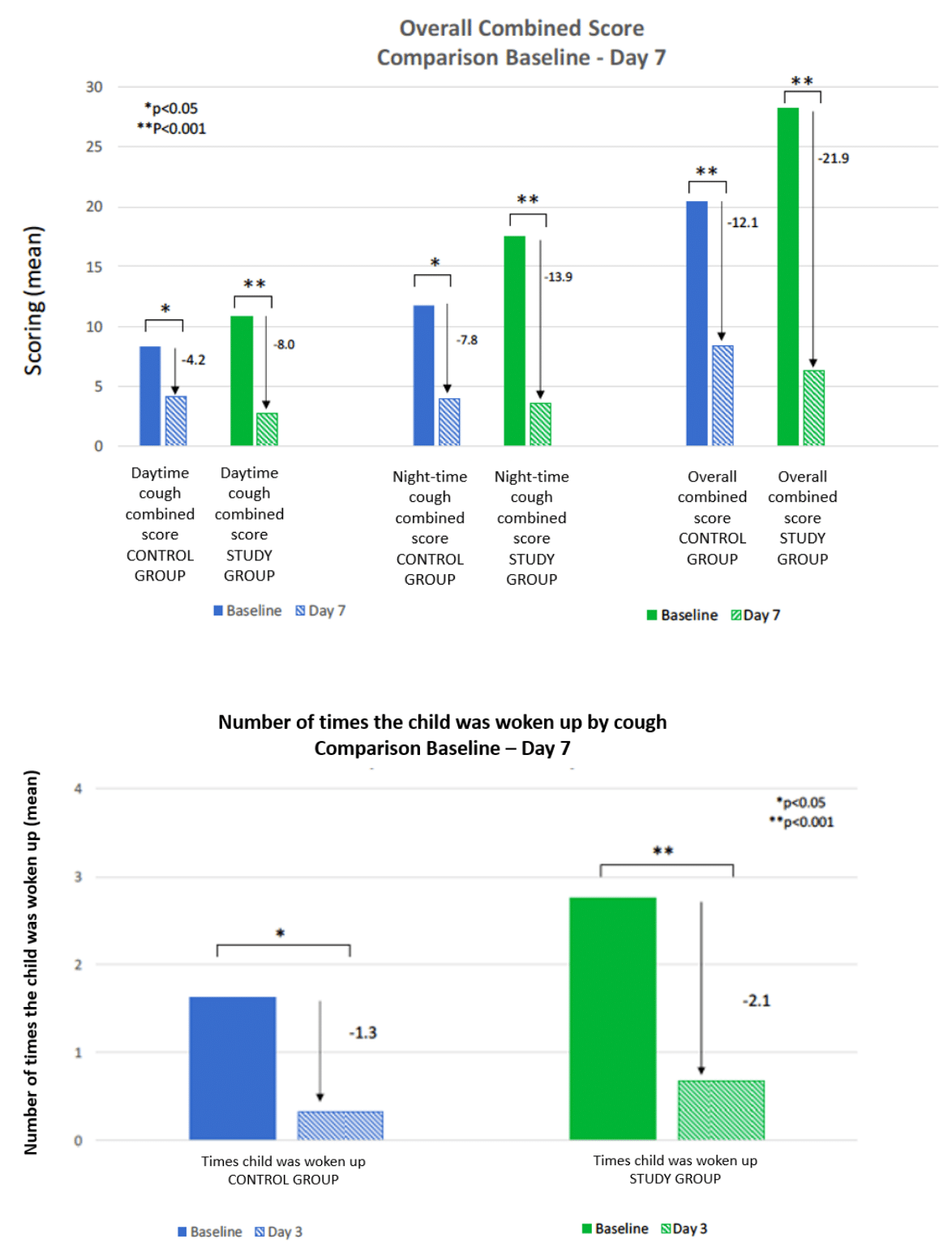
Figure 2 Comparison baselines -Day 7.
A) Overall Combined Score.
B) Number of times the child was woken up by cough.
The number of times patients were awakened by coughing on Day 7 significantly decreased in both groups. However, the reduction was more pronounced and statistically significant in the study group compared to the control group.
Patient diary data shows that both groups experienced a gradual decrease in night-time cough over time (Figure 3). The study group exhibited a faster reduction in each symptom compared to the control group. This trend was observed from baseline and continued until Day 7 for all symptoms, except for the affectation of the parent's ability to sleep, which showed an accelerated reduction until Day 6.
The results of the degree of satisfaction with the investigational device show that the majority of physicians and parents had positive feedback (Table 3). Specifically, 32.10% of physicians (9 out of 28) reported being "very satisfied" and another 28.6% (8 physicians) were "moderately satisfied". Among parents, 27.6% (8 out of 29) expressed being "very satisfied", while 24.1% (7 parents) felt "satisfied". It is worth noting that the number of respondents who had negative feelings (from "very unsatisfied" to "moderately dissatisfied") about the device was comparatively low, especially among physicians.
|
Satisfaction with the investigational device |
Physicians* |
Parents |
||
|
N |
% |
N |
% |
|
|
Very unsatisfied |
1 |
3.57 |
2 |
6.9 |
|
Dissatisfied |
0 |
0 |
2 |
6.9 |
|
Moderately dissatisfied |
0 |
0 |
1 |
3.4 |
|
Neither satisfied nor dissatisfied |
3 |
10.7 |
4 |
13.8 |
|
Moderately satisfied |
8 |
28.6 |
5 |
17.2 |
|
Satisfied |
7 |
25 |
7 |
24.l |
|
Very satisfied |
9 |
32.1 |
8 |
27.6 |
|
All |
28 |
100 |
29 |
100 |
|
*Data is not available for 1 case |
||||
Table 3 Degree of satisfaction with investigational device
Safety
No serious adverse events were reported during the study. Two patients (one in the study group and one in the control group) required withdrawal from the study due respiratory complications not causally related to the investigational medical device or the clinical investigation. Two patients in the study group reported adverse events which might have a causal relationship with the investigational medical device: sleepiness and more frequent and soft bowels that may be due to presence of sorbitol that could have laxative effects. In both cases, no action was taken, and they resolved on their own without the need to stop treatment.
The study findings indicate that AJCS, a natural mucilage-based cough syrup with organic essential oils, reduces cough associated with acute upper respiratory infections in children more effectively and rapidly than hydration measures and maintains a safety profile.
This study relies on the quantification of patients' subjective perception and verbal expression of cough as a means to measure the impact of cough on children. The reliability of this method in assessing the effectiveness of cough treatments has been previously validated and widely utilized in the scientific literature.26,27 Furthermore, to obtain a comprehensive understanding of the treatment's effect, a cough diary and satisfaction questionnaire were administered to both pediatricians and parents.28 This allowed for a comprehensive assessment of the treatment's impact.
During the baseline assessments, it is significant to emphasize that the study group demonstrated elevated levels of daytime cough frequency and severity, night-time cough severity, impairment of the child’s sleep, and overall combined cough scores when compared to the control group. The variance in baseline characteristics could be attributed to the allocation method employed, which relied on the clinician’s discretion. One possible explanation for this outcome is that, due to the control group’s absence of drug treatments, pediatricians may have opted to administer AJCS to patients with more severe or frequent cough symptoms. Taking this factor into account when interpreting the results is crucial since it introduces a baseline bias that hinders a direct comparison of the progression of cough symptoms between the two groups.
On Day 3, the study group showed greater reductions in combined scores for daytime cough, night-time cough, and overall cough compared to the control group. Although there were no significant differences in combined cough scores between the two groups, the improvements were more significant in the study group when considering raw score differences and percent changes. Both groups experienced significant improvement in daytime cough, but significant improvements in night-time cough and overall cough score were observed only in the study group. Additionally, the study group had a greater decrease in the number of times patients woke up with a cough on Day 3, which was significant only in the study group, not in the control group. These findings suggest that the tested product may be more effective than hydration measures in reducing cough severity, particularly during the first three days, with a specific impact on night-time cough. This aspect of AJCS is particularly important as night-time cough poses significant challenges and is difficult to manage.29 By mitigating the severity of night-time cough, there is potential to enhance the overall quality of life, as this symptom is recognized to cause substantial discomfort and disrupt sleep for both children and parents.30–32 Moreover, night-time cough problems present multiple challenges.29 Firstly, disrupted sleep can have a cascading effect on overall health and well-being.33 Adequate sleep is crucial for the body's restorative processes, and frequent awakenings due to coughing can result in fragmented and poor-quality sleep.34 This can lead to daytime fatigue, decreased cognitive function, and difficulty concentrating.35 Finally, individuals with night-time cough may experience increased anxiety or stress due to the anticipation of coughing episodes, further aggravating sleep disturbances.36 In conclusion, the management and control of night-time cough should be considered a crucial aspect when evaluating the effectiveness of a cough treatment. Further investigations are warranted to shed more light on and confirm the ability of AJCS to reduce night-time cough in children.
Regarding the results obtained at Day 7, significant changes were observed. The study group had lower cough assessment scores and combined scores compared to the control group, which had higher scores at baseline. Both groups experienced a significant decrease in all combined scores on Day 7, but the study group showed even greater reductions in daytime cough, night-time cough, and overall combined score compared to the control group. Additionally, the study group had a more significant reduction in the number of times patients woke up coughing on Day 7, highlighting the superior efficacy of the tested product compared to hydration measures. The study product’s effectiveness from the first seven days of treatment is particularly noteworthy, especially considering that parents may start to worry after one week of symptoms and seek alternative interventions for their child.8,37 The pursuit of alternative therapies can expose children to potential risks associated with receiving inappropriate treatment.8,37 Therefore, the need for fast relief becomes crucial when considering cough treatment options.
The analysis of the patient diary data also revealed that the study group showed a faster reduction in each symptom compared to the control group, except for the affectation of the parent's ability to sleep, which improved more rapidly until Day 6. These findings provide additional evidence of the superior efficacy of the tested product compared to hydration measures, as it leads to a quicker improvement in symptoms.
Regarding the study of adverse events, the findings of the present study provide evidence for the safety of the study product. No serious adverse events were reported during its use, and there were no device deficiencies observed. The safety analysis conducted also supports the product's safety profile, with no observed toxicity in children.
In addition, the satisfaction questionnaire not only reinforces the effectiveness and safety of the tested product but also highlights the importance of patient compliance in the overall efficacy of cough treatment. Previous studies have shown that parental satisfaction with a medication is often linked to better treatment adherence.38,39 Therefore, the data obtained from the questionnaire in this study suggests the potential for high treatment compliance, which is a crucial factor in achieving optimal outcomes in cough management.40
The findings of this study should be considered in light of certain limitations associated with its design. It is important to note that patient allocation was not randomized, leading to significant differences in baseline cough symptoms between the control and study groups. Such variations in initial conditions introduce a baseline bias, complicating the direct comparison of cough symptom progression between groups.
In conclusion, this study provides further evidence supporting the efficacy and safety of AJCS (Aromaforce® Junior Cough Syrup) in the treatment of cough associated with acute upper respiratory tract infections in children. The results demonstrate that AJCS effectively reduces cough severity, particularly within the first three days and during night-time episodes, surpassing the effectiveness of hydration measures. Furthermore, the safety analysis confirms the syrup's non-toxic nature in children. However, the initial differences in baseline characteristics between the study and control groups, with the study group exhibiting higher combined cough scores, limit the strength of the evidence. Further randomized clinical trials may provide a clearer understanding of the medical device’s efficacy, as current results are promising but lack statistical significance.
Grateful acknowledgment is extended to Crossdata, a Contract Research Organization (CRO), for their invaluable collaboration in conducting the study and their significant contribution to the drafting of this article.
This study was conducted with the financial support from Pranarȏm España S.L.U.
We have no conflict of interests to declare.

©2024 Pacheco, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.