Journal of
eISSN: 2373-4426


Case Report Volume 13 Issue 1
Pediatric Clinic, Clinical Center University of Sarajevo, Bosnia and Herzegovina
Correspondence: Halimic Mirza, Department of Cardiology, Pediatric Clinic, Clinical Center University of Sarajevo, Bosnia and Herzegovina, Tel +387 61 217 557
Received: February 09, 2023 | Published: April 4, 2023
Citation: Halimic M, Kadic A, Begic Z, et al. Case report: right sided endocarditis in pediatric patient after surgical treatment of tetralogy Fallot. J Pediatr Neonatal Care. 2023;13(1):44-45. DOI: 10.15406/jpnc.2023.13.00489
Right-side endocarditis is a well-defined clinical entity, rarer than left-side endocarditis. Infective endocarditis in children has multiple challenges. A history of congenital heart disease is the most common risk factor, although other emerging predisposing conditions have important relevance. We report the clinical presentation, diagnosis and management in a pediatric patient with isolated native pulmonary valve endocarditis one year after surgical treatment of Tetralogy Fallot.
Keywords: endocarditis, pulmonary regurgitation, tetralogy of Fallot, ventricular septal defect
Right-sided endocarditis encompasses approximately 12% of all cases of infective endocarditis (IE), with the tricuspid valve being the predominant site of infection. Involvement of the pulmonary valve (PV) alone is unusual and often occurs in the presence of congenital heart disease. We report the clinical presentation, diagnosis and management in a pediatric patient with isolated native PV endocarditis one year after surgical treatment of Tetralogy Fallot.
On admission, a female child aged 1.5 years, TM 9300g, subfebrile, extremely pale, adynamic, slightly tachypneic, hypotrophic, with hursh heart murmur on the left sternal border. Present indicators of anemia and thrombocytopenia in the blood count. With elevated CRP. Staphylococcus epidermidis was isolated in three repeated blood cultures. Antibiotic treatment was started (Ampicillin, Amikacin, later changed for Meropenem and Vankomicin). In the second week of hospitalization, extensive gastrointestinal bleeding, which was treated with conservative medication measures.
Preoperative echocardiography
The ventricles are balanced. The patch placed on the defect in the perimembranous-outlet region in the interventricular septum was seen as echogenic, a left-to-right shunt from the 3mm residual defect at the upper end of the patch and a 38mmHg gradient between the two ventricles was detected large, lobulated echogenic and mobile mass was seen in the pulmonary valve. Measured 12-15mm in diameter. It was determined that it extended towards the right pulmonary artery with a thin pedicle and the length of the extension was 17mm. A 32mmHg gradient was measured at the valve level, and mild insufficiency was determined. There is degree 2 insufficiency of the tricuspid valve. Flow velocity was measured at 3.8m/s (58mmHg). RV pressure has been estimated at 60-65mmHg. The aortic valve has three cusps, with minimal regurgitation. There is mild (1st degree) regurgitation from the mitral valve. Thrombus/vegetation was not observed in other vessel cavities on tricuspid, mitral and aortic valves. Coronary artery outputs are normal. Left ventricular function is normal. RF about 65% here is no pericardial effusion (Figure 1 & 2).
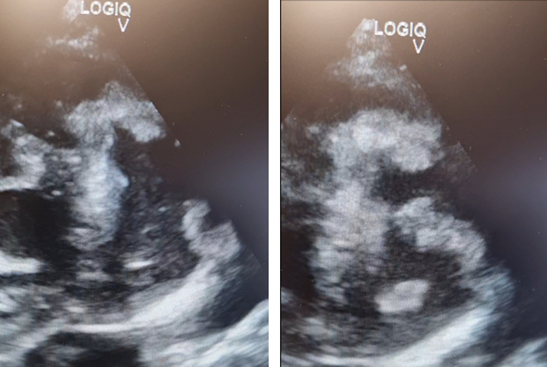
Figure 1 Transthoracic echocardiogram: A parasternal, short-axis view shows a mobile mass consistent with vegetation adjacent to the pulmonary valve.
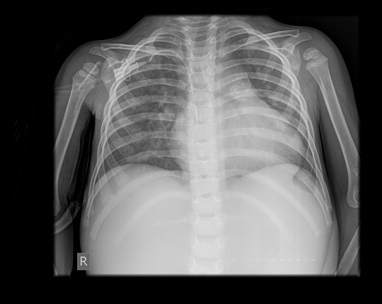
Figure 2 X ray-bronchovascular drawing emphasized right hiloperihilar and paracardiobasal with a field of plate-like small atelectasis.
Surgical procedure
VSD closure, pulmonary valve resection, excision of infected tissues.
Postoperative course: The patient was transferred to ICU within stable hemodynamic findings with moderate inotropic support. The patient was extubated an early after operation and transferred to the patient floor on the first postoperative day. No microorganism was isolated in the tissue culture sent during the operation. Antibiotic treatment was continued for two weeks postoperatively. The last post-ICU follow-up was uneventful and the last chest X-ray and laboratory findings were within normal limits.
About 50–70% of pediatric IE is seen in patients with congenital heart disease, which is the main predisposing condition.1 Cyanotic and complex congenital heart disease, left-sided defects, or endocardial cushion defects are the main variants associated with higher risk.2–4 Recent reconstructive cardiac surgery (<6 months) in patients with congenital heart disease is an important predisposing factor for IE.5–7 Causal pathogens vary according to the underlying conditions.6–9 Even there have been changes in IE epidemiology in recent decades, S. aureus remains the dominant causative pathogen both in children and adults, Coagulase-negative Staphylococci was isolated in 10–15% patients with congenital heart defects.1,7 Patients with tetralogy of Fallot (ToF) are considered at high risk of infective endocarditis (IE) as a result of altered hemodynamics and multiple invasive procedures.10 Tetrology of Fallot (TOF) is the most common form of cyanotic congenital disease and has had marked improvement in outcomes over the last decades with advancements in medical and surgical repair. Nevertheless, TOF repair is still associated with a number of postsurgical complications.11 In right-sided endocarditis, surgery indications and its timing are much less clear than in left-sided infections, but current literature describes it as associated with a significant morbidity, mortality, and high likelihood of requiring surgery. Large vegetations and clinical signs of haemodynamic impact should prompt consideration of early surgical intervention.12
None.
None.
The author declares no conflicts of interest.

©2023 Halimic, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.