Journal of
eISSN: 2373-4426


Background: Intravenous fish-oil-based fat emulsions enriched with omega 3 fatty acids, such as Omegaven, are available through investigational new drug (IND) and emergency IND (eIND) applications to ameliorate parenteral nutrition associated liver disease (PNALD).
Objective: To describe changes in conjugated, direct bilirubin in the cohort of preterm neonates before and after continuous Omegaven administration.
Design/Methods: Between April 2009 and July 2011, under compassionate use protocols at St Louis University/Cardinal Glennon Children’s Medical Center and George Washington University/Children’s National Medical Center, preterm neonates receiving parenteral nutrition (PN) with soybean emulsions were treated with Omegaven and prospectively followed. Eligibility requirements included serum direct bilirubin levels ≥ 3 mg/dL and predicted PN duration of > 21 days. Omegaven stopping criteria were either the serum direct bilirubin level < 1 mg/dL or sufficient tolerance of enteral nutrition, prompting PN discontinuation. To assure sufficient Omegaven therapeutic exposure, only neonates receiving Omegaven for ≥ 30 days were included in the data analysis. Wilcoxon signed-rank test was used to assess serum direct bilirubin change.
Results: Data of 17 very low birth weight preterm neonates were analysed: over a median of 64 days of Omegaven therapy, serum direct bilirubin level decreased significantly (6.16 mg/dl vs 4.82 mg/dl, p=0.035). Importantly, followed safety markers were unchanged during therapy.
Conclusions: Modest, yet statistically significant decrease in serum direct bilirubin levels was associated with fish-oil based lipid emulsions use in our cohort of cholestatic preterm neonates.
Keywords: cholestasis; preterm neonate; omegaven; safety
PN, parenteral nutrition; PNALD, parenteral nutrition associated liver disease
Neonates with inadequate bowel function become parenteral nutrition (PN) dependent for their necessary fluid and nutritional requirements. Parenteral nutrition-associated liver disease (PNALD) develops in some of these neonates leading to severe liver failure. Currently, there are limited therapies to treat this condition. Experimental data suggest that the soybean based intravenous lipid preparation used may contribute to the development of PNALD. Omegaven, a fish-oil derived parenteral lipid emulsion rich in omega-3 fatty acids, has been shown in case studies to limit or reverse the progression of PNALD.1 Omegaven was approved by the FDA for use in the study institutions in a compassionate use protocol under an Investigational New Drug application.
Our objective was to evaluate the safety profile and changes in serum direct bilirubin levels in preterm neonates with moderate to severe PNALD treated with Omegaven. A prospective observational study was performed on preterm neonates receiving Omegaven for more than 30days. The protocol was IRB approved by the institutions and parental informed consent was obtained prior to Omegaven treatment. We included infants who were PN dependent for at least 3weeks, had 2 consecutive serum direct bilirubin levels of >3mg/dl (separated by at least 7days), failed standard therapies to prevent progression of liver disease and were expected to receive PN for at least 3 more weeks. Neonates with other etiologies of liver dysfunction, active coagulopathy or impaired lipid metabolism or severe hyperlipidemia were excluded.
Omegaven was the only lipid infusion provided during the study period, initiated at 0.5gm/kg/d for 2days, and then increased to 1gm/kg/d for the remainder of the treatment course; biochemical markers and growth parameters were obtained at baseline and weekly after initiation of therapy. Criteria of Omegaven cessation were 2 consecutive serum direct bilirubin levels < 1mg/dl or tolerance of enteral feedings adequate to stop parenteral nutrition. Change in serum direct bilirubin was compared using the Wilcoxon signed-rank test.
Total of 21 infants were administered Omegaven under compassionate protocol use, 4 infants were not included in the final analysis (3 received Omegaven for less than 30days, 1 received Omegaven inconsistently). Seventeen neonates included in the analysis had mean gestational age at birth of 26.6 weeks, mean birth weight of 911 gm and had Omegaven administered for a median of 64days. The main finding of our study was statistically significant decrease of serum direct bilirubin level from initiation therapy (6.16 mg/dl) to cessation of therapy (4.82mg/dl), p = 0.035. Individual patient response characteristics are illustrated in Figure 1.
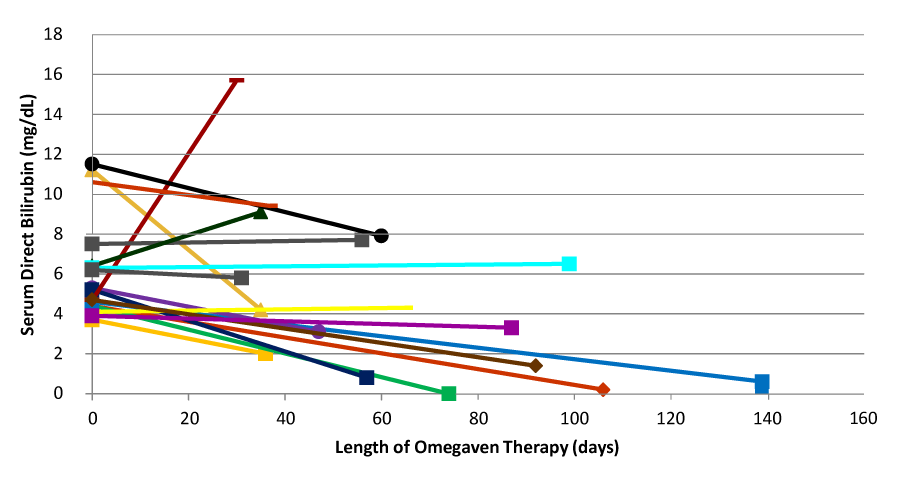
Figure 1 Changes in Mean Facial (± S.E.) Width-to-Height Ratios of Major League Baseball Players Active 1870-1989.
The neonates tolerated Omegaven well, without significant changes in the safety indicators followed (Table 1). No clinically significant bleeding related to Omegaven was noted in any subjects. This safety profile, as well as the reduction in direct bilirubin, is comparable to previous reports.1,2 Limitations of our study are lack of standard randomization and the fact that lipid loads for soybean oil (Intralipid) were not constant across the study subjects. Since the fish oil administration was limited to the dose 1gm/kg/day, it remains unclear, whether the cholestasis improvement was due to the different type of lipid, overall lipid dose reduction,3 or both. Future trials should not only answer these questions related to cholestasis reversal, but should also focus on cholestasis prevention. Lipid emulsions containing mixture of different oils appear to be one promising therapeutic approach.4,5
|
Biochemical marker |
Value |
|
INR (International Normalized Ratio) |
1.2 (1.0, 2.0) |
|
Platelet Count (K/mm3) |
161 (75, 587) |
|
Serum Albumin (g/dL) |
2.6 (2.1, 3.8) |
|
Maximum serum triglyceride (mg/dl) |
94 (52, 210) |
Table 1 Safety indicators during omegaven treatment course [median (min, max)]
None.
The authors have no conflict of interests related to this publication and have not received any grants.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 International Childhood Cancer Day is observed on 15 February 2026 to raise awareness about childhood cancers and to highlight the medical, developmental, and supportive care needs of affected children and their families. This day emphasizes the importance of early diagnosis, pediatric care, and continued research to improve survival and quality of life in children with cancer.
Researchers and healthcare professionals are encouraged to submit their original research articles, reviews, and clinical studies related to pediatric oncology, neonatal care, and child health. Manuscripts submitted on the occasion of International Childhood Cancer Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).
.
International Childhood Cancer Day is observed on 15 February 2026 to raise awareness about childhood cancers and to highlight the medical, developmental, and supportive care needs of affected children and their families. This day emphasizes the importance of early diagnosis, pediatric care, and continued research to improve survival and quality of life in children with cancer.
Researchers and healthcare professionals are encouraged to submit their original research articles, reviews, and clinical studies related to pediatric oncology, neonatal care, and child health. Manuscripts submitted on the occasion of International Childhood Cancer Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).
.