Journal of
eISSN: 2373-6445


The hippocampus plays a central role to form new episodic memory in various species including humans.1 The hippocampal neurons seem to process variety of information, such as spatial location,2 temporal information,3 and emotional state4 within specific episodes.5 However, the basic rules such as how to sustain a piece of specific memory and what associates the memory fragment each other are completely unknown.
Stressful experience sometimes forms strong episodic memory in the hippocampus, which is useful to avoid similar risk in future. As a learning paradigm, we used an inhibitory avoidance (IA) task. In the task, we placed a rat in an illuminated box. When the rat entered into the dark side of box, we applied brief electrical foot shock in the dark side. Even one-time foot shock experience, the rat quickly learns the experienced episode. The rats were returned to the home cage, then 30 min after, we placed the rat in the illuminated box again. We measured the latency in the illuminated box as a learning performance (Figure 1). After the training, we made acute brain slices to analyze synaptic function using slice patch-clamp technique.
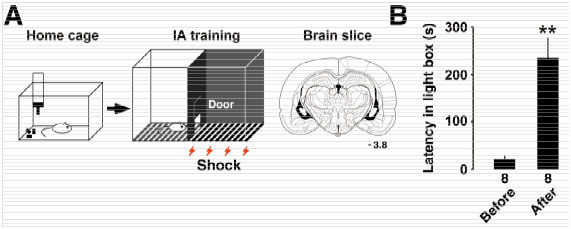
Figure 1A Schema of inhibitory avoidance (IA) task. Rats were experienced an episode with an electrical foot shock. B: After the experience, the rats showed longer latency in the illuminated side of the box.8
Although some aspect of the training-induced synaptic plasticity6 is similar to the tetanus stimuli-induced LTP,7 we found many novel aspects of plasticity in trained animals. For example, to analyze the plasticity, we recorded the postsynaptic response induced by single vesicle of GABA (mIPSC) or glutamate (mEPSC) in the presence of a Na+ channel blocker (Figure 2A). The means of mEPSC amplitude and mIPSC amplitude were calculated in each neuron, and the data was plotted in Figure 2B. Although the amplitudes were low and showed a narrow distribution range in untrained, unpaired, or walk-through rats, IA training not only strengthened AMPA receptor-mediated excitatory inputs, but also increased GABAA receptor-mediated inhibitory synaptic inputs in CA1 neurons (Figure 2). These results suggest that the training promoted the diversity of excitatory and inhibitory inputs in CA1 neurons. IA training enhanced the diversity of postsynaptic excitatory/inhibitory responses in CA1 neurons. Question arises as to whether the learning requires the synaptic plasticity. We found bilateral CA1 expression of GluA1-c-tail or MPR-DD, an AMPA receptor-delivery blocker, successfully impaired the learning,9 suggesting the contextual learning requires the synaptic delivery of GluA1 containing AMPA receptors. Moreover, recent chromophore-assisted light inactivation technique (CALI) further demonstrated that optical inactivation of synaptic AMPA receptors can erase acquired memory.10 These results further showed that newly delivered GluA1-containing AMPA receptors contribute to forming contextual memory.
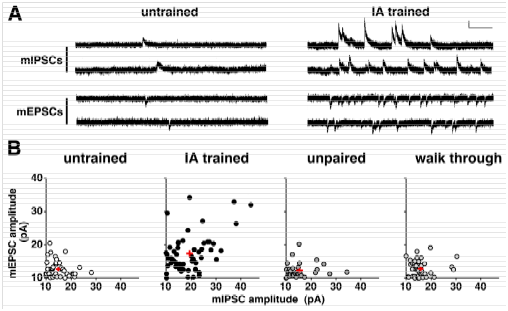
Figure 2A & 2B Examples of miniature postsynaptic response induced by 1 synaptic vesicle of glutamate (mEPSC) or GABA (mIPSC). Vertical bar = 20 pA horizontal bar = 200 ms. B: The amplitude of mEPSCs or mIPSCs was averaged and plotted. One plot represents the data from a single CA1 neuron.
As to the inhibitory synaptic plasticity, nicotinic a7 receptor antagonist is known to block the strengthening of GABAA receptor-mediated synapses.8,11 Since pharmacological blockade of the plasticity in bilateral CA1 impaired the learning, contextual learning seems to require the inhibitory synaptic plasticity in the CA1. Moreover, optogenetic enhancement and suppression technique further revealed the role of the GABAergic inhibitory synapses in learning.12 Taken together, I hypothesized that the contextual learning requires the training-induced synaptic plasticity at both excitatory and inhibitory synapses in CA1 (Figure 3).
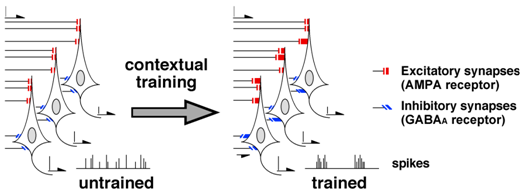
Figure 3 Current hypothesis of learning & memory. Contextual training induces diversity of the synaptic inputs in CA1 neurons. Since GABAergic inputs are critical to synchronize the spike firing,29 the multiple CA1 neurons probably form diversified ripple-like events after training. Interestingly, all ripples or ripple-like events in many laboratoly groups show different shape.25‒28
The learning-induced synaptic diversity can be analyzed mathematically. By calculating the appearance probability of each point of Figure 2B, data from each neuron can be converted to self-entropy (bit) using the information theory of Ralph Hartley19 and Claude E Shannon.13 A point with high appearance probability (around the mean level) indicates low self-entropy, while a point with very rare probability (a deviated point) indicates high self-entropy. Compared with untrained rats, the self-entropy per neuron was clearly increased in IA-trained rats but not in unpaired or walk-through rats.8 This analysis suggests a learning-induced increase in the amount of information in CA1 neurons. Although each neuron showed different self-entropy level, we calculated self-entropy level per single CA1 neuron after the training.14 By multiplying 4.0 x 105, the total number of CA1 neurons in rats,15 we could estimate a possible amount of intra-CA1 self-entropy after the training.
Synaptic inputs regulate neuron firing according to the all-or-none principle.16 As neurons are considered an all-or-none device,17,18 one neuron can handle 1-bit of memory per clock cycle (log2 2 = 1 bit) according to a principal formula of information theory.19 Based on the principle, computational theory proposed a role of the hippocampus as a kind of memory device.20 Our previous finding of a logarithmic correlation between the number of cells blocking plasticity and learning performance9 may provide evidence that each CA1 neuron transmits binary data, forming a contextual memory, such as what, where, or when.
Since many diverse features contain entropy, the synapse is not a solo factor to be analyzed. For example, 3.1 x 109 base pairs21 of the human genome quaternary system22,23 are identical to 6.2 x 109 bits in a binary system. However, 6.2 x 109 bits contain only 755 megabytes (1 byte = 8 bits), the amount is not more than one hour of short movies. Although postnatal maternal care affect the offspring epigenetic programming,24 postnatal experience cannot affect the genomic quaternary information. Because our brains process much more information per day, an understanding of genome information cannot be sufficient to reveal the learning systems.
The diversified excitatory/inhibitory synapses after training may create the diversity of ripple-like on/off firing in hippocampal CA1 (Figure 3). In fact, hippocampal ripple-like events seem to exhibit diverse features25,26 and phase-locked (≈ 180°) with theta waves.27 Interestingly, all the published ripple-like events from many research groups were not identically same. The ripple-like firing may code experienced information, as selective suppression of hippocampal ripples impairs spatial memory in dorsal CA1.28 Moreover, we previously found that contextual learning requires the plasticity at excitatory/inhibitory synapses in CA1.8,9 Our results, together with the previous studies, we hypothesized that the excitatory/inhibitory synaptic plasticity creates the ripple-like events to process the experienced context by using the theta phase-locked processing system. We are going to analyze the diversity of the ripple-like events in the CA1 in trained animals, that would be necessary to decode intra-CA1 information.29
None.
Author declares there are no conflicts of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.