Journal of
eISSN: 2379-6359


Case Report Volume 13 Issue 2
Department of Otolaryngology, Head and Neck Surgery, Brazil
Correspondence: Bárbara Cecília Borges Moreira, Department of Otolaryngology, Head and Neck Surgery, Santa Casa BH Group, Minas Gerais, Brazil, Tel +55 31991165985
Received: March 09, 2021 | Published: March 26, 2021
Citation: Moreira BCB, Azevedo AF, Castro MCM. Spontaneous temporal cerebrospinal fluid leak. J Otolaryngol ENT Res. 2021;13(2):22-27. DOI: 10.15406/joentr.2021.13.00485
Spontaneous cerebrospinal fluid leaks of the temporal bone are uncommon conditions, but with increasing incidence in the last years. They represent the osteodural defect of the middle and posterior fossae floor with consequent communication between the subarachnoid space and the middle ear and mastoid cells, not associated with a history of trauma, chronic infections, tumors, surgery or irradiation. Physiopathogenesis is not well defined, being associated with the faulty development of the temporal bone and/or the presence of aberrant arachnoid granulations, in addition to being favored by idiopathic intracranial hypertension, obstructive sleep apnea and obesity. It has the potential for serious neurological complications, which is why surgical treatment is recommended. The main approaches involve transmastoid access and craniotomy access through the middle fossa, or a combination of both. This paper reports on a case attended at Grupo Santa Casa BH and performs a literature review and on aspects related to the clinical presentation and management of the condition.
Keywords: cerebrospinal fluid leak, cerebrospinal fluid otorrhea, middle cranial fossa, posterior cranial fossa
Spontaneous cerebrospinal fluid leaks (CSF) of the temporal bone are uncommon conditions, but with an increasing incidence in recent years and, therefore, knowledge of their pathophysiology, presentation and treatment are fundamental for good otorhinolaryngological practice. They represent the manifestation of the defect of the middle and posterior fossa floor with consequent communication between the subarachnoid space and the middle ear and mastoid, not associated with a history of traumatic brain injury, chronic infections, tumors, surgery or irradiation.1–3 They may be due to the failed development of the temporal bone or the presence of aberrant arachnoid granulations1,3,4 and are favored by idiopathic intracranial hypertension, obstructive sleep apnea and obesity.5–10 Temporal CSF leaks may also be associated with the presence of herniations of the intracranial content (meningoceles and meningoencephaloceles) and with dehiscence of the superior semicircular canal.11–13
The clinical presentation is usually insidious and nonspecific, which tends to delay the diagnosis. The most common signs and symptoms are effusion in the middle ear, aural fullness, dysacusis, tinnitus and clear, pulsatile and persistent otorrhea after placing a ventilation tube.2,13 However, in some patients, the initial clinical manifestation can be meningitis, brain abscess and seizures, complications associated with spontaneous CSF leaks that impose the need for imperative surgical treatment.2,11,13,14 The potential sites involved in temporal CSF fistulas are the tympanic tegmen, the mastoid tegmen, mastoid, petrous apex, Hyrtl's tympanomeningeal fissure, facial nerve canal and stapes platinum. Therefore, the anatomical domain of the temporal bone is essential for proper identification and management of the condition. Computed Tomography (CT), Nuclear Magnetic Resonance (NMR) and measurement of β2 transferrin are the main complementary exams for investigation and diagnosis.13 The main surgical techniques for the correction of temporal bone dehiscence and dural defect are transmastoide approach, middle fossa craniotomy or the combination of both, with the possibility of using autologous or synthetic grafts and combinations of these.1,4,13 In addition, it is necessary to manage comorbidities associated with the development of spontaneous CSF leaks to avoid persistence and recurrences.6,7,9,15
A 42-year-old male patient, with a body mass index (BMI) of 28.7 kg/m², previously healthy, with no relevant otorhinolaryngological history, reported sudden aural fullness and hearing loss in the right ear. He denied tinnitus, vertigo, otalgia, otorrhea or nasosinusal and pharyngolaryngological complaints. After maintaining the symptoms for a few weeks, he sought otorhinolaryngological assistance, and presented, on physical examination, with otoscopy showing tympanic membrane hyperemia. Audiometry and immittance tests were performed, which showed moderate conductive hearing loss (average of 58dB and bone air gap of 40dB) and type B tympanometric curve, respectively (Figure 1); Nasofibroscopy that did not show significant changes in nasal cavities and rhinopharynx; and laboratory tests for serological screening, all with negative results. A diagnosis of serous otitis media was made and drug treatment with oral antibiotics and corticosteroids was instituted as an attempt to resolve the condition. The patient did not obtain a satisfactory response, and tympanotomy for placement of a ventilation tube was indicated. During the surgical procedure, clear serous secretion in the middle ear was verified. After placing the ventilation tube, the patient maintained persistent fluid otorrhea (Figure 2).

Figure 1 Preoperative audiometry.
There is a moderate conductive hearing loss in the right ear and a normal hearing threshold in the left ear.
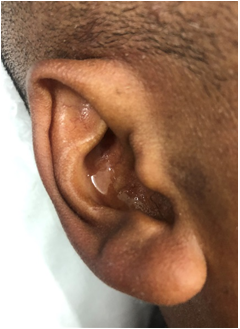
Figure 2 Patient's clinical presentation.
Clear fluid otorrhea maintained after tympanotomy and ventilation tube placement.
A CT of the temporal bone was performed and showed only the partial veiling of the middle ear and mastoid cells by material with soft tissue density (Figure 3). Then, the secretion was collected for glucose measurement (since the measurement of β2 transferrin was not available), which suggested CSF. A new CT of the ears was performed, and this time with identification of a small bone defect in tympanic tegmen.
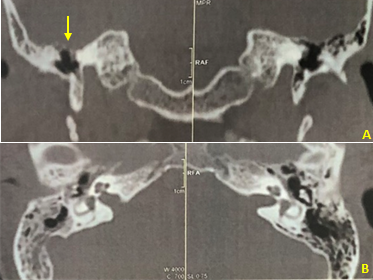
Figure 3 Preoperative Computed Tomography of temoral bone.
A. Coronal section. B. Axial section. There is a tympanic tegmen bone defect and partial veiling of the middle ear and mastoid cells.
Before confirmation of the diagnosis of spontaneous temporal CSF leaks, the patient was referred for anti-pneumococcal vaccination and submitted to a surgical approach by the middle fossa craniotomy by the Otorhinolaryngology and Neurosurgery teams at Hospital São Lucas, belonging to the Santa Casa BH Group (Figure 4). During the procedure, multiple small dehiscences in tympanic and mastoid tegmens were observed, as well as a small meningocele with extension to the middle ear. Multilayer closure performed with bone wax, dural substitute, temporal fascia, biological glue and bone graft. In the postoperative follow-up, the patient showed complete improvement in complaints, as well recovery of hearing thresholds and good aeration of the middle ear and mastoid cells on complementary exams (Figures 5 & 6).
CSF leaks are the result of a discontinuity in the arachnoid, dura mater, bone and underlying mucosa that allows the passage of cerebrospinal fluid to the extradural sites, either in the nose or ear, causing rhinoliquorea or otoliquorrhea, respectively.16 Usually, otological CSF leaks are related to a history of temporal trauma, chronic otitis, neoplasms, otological or neurological surgeries or irradiation.1–3
The existing theories for the pathophysiology of spontaneous fistulas are related:
A recent data survey shows that the average age of patients diagnosed with spontaneous temporal CSF leaks is 56.9 years, ranging from 45.7 to 63 years.3,6,8–10,17,19–34 The patient's age in this report is, in a way, consistent with the hypothesis that spontaneous temporal CSF leak is associated with years of pulsation of intracranial content in regions of thin bones due to increased pneumatization, dehiscence or arachnoid granulations presence of the middle fossa floor. Regarding gender, most studies have noted a higher proportion of women to men in the development of spontaneous temporal CSF leaks, while others have not observed this trend.2,3,6,8,19–22 Our study presents a case in a male patient. The reason why women are more affected by spontaneous CSF fistulas of the temporal bone is not understood, but it can be associated with a higher incidence of idiopathic intracranial hypertension (4:1) and pregnancies21. About the laterality, the predominance of the right side was observed in several studies, which is in accordance with the clinical case reported.3,17,20,23–26 In this regard, it is worth mentioning the frequency of bilateral cases, even if they do not develop at the same time, since pathophysiological mechanisms tend to act bilaterally. Studies point to an average of 10.2% of bilateral cases.6,8,10,17,20–23,28
In addition, spontaneous CSF leaks may be predisposed by clinical conditions, such as obesity, obstructive sleep apnea syndrome and idiopathic intracranial hypertension. These conditions are associated with a chronic increase in intracranial pressure, contributing to the development of bone erosions by increasing the the intracranial content pulsation pressure against the skull base with its consequent erosion.5–9,35 Due to the fact that they are often mutually favored and, therefore, often present in the same patient, the increase in their incidences in the last decades may justify the increase of the spontaneous CSF leaks incidence in the period. Data published by the Centers for Disease Control (CDC) have estimated that 39.8% of United States adults were obese in 2015–2016, compared with 30.5% in 1999–2000, and in the 10-year period between 2002 and 2012, while there was no change in the repair of secondary CSF leaks rate, the repair of spontaneous temporal CSF leaks rate increased by 124%.7 In another study, have been demonstrated that the spontaneous CSF leaks patients BMI was significantly higher than the secondary CSF leaks patients BMI.4,9
Studies have demonstrated that 14.4 to 16.9% of spontaneous CSF leaks patients have sleep apnea syndrome and that the risk of developing CSF leak is 4.7 times higher in those.4,7,36 Studies show that 72% of spontaneous CSF leaks patients meet the modified Dandy criteria for the idiopathic intracranial hypertension diagnosis (a. Signs and symptoms of increased intracranial pressure such as papilledema, visual disturbance, pulsatile tinnitus and headache; b. Normal neurological examination; c. Increased intracranial pressure documented by lumbar puncture performed in lateral decubitus, with normal CSF composition - greater than 20 cmH20 in non-obese patients and greater than 25 cmH20 in obese patients; d. Absence of neuroradiological exams alterations - except for small ventricles and empty sella; e. Absence of other identifiable causes of intracranial hypertension).15 The patient who motivated this report was overweight (BMI between 25-30 kg / m2), a favorable situation for CSF leak development. However, he did not have snoring or hypopnea/apnea and did not meet criteria for idiopathic intracranial hypertension.
Through the temporal bone defect, intracranial content herniations to the middle ear and to the mastoid may occur, together or not, with spontaneous CSF leaks, characterizing meningoceles and meningoencephaloceles. A recent survey has showed that 64% of the spontaneous CSF leaks are associated with herniations.3,8–10,17,18,20,22–24,26,29,30,32,37 In addition, the pathophysiology of spontaneous CSF leaks, due to anatomical issues, is associated to superior semicircular canal dehiscence. In patients with spontaneous temporal CSF leaks, approximately 13.1% will be associated to superior semicircular canal dehiscence.10,17,24,27,28 The clinical presentation is usually insidious and nonspecific, which tends to delay the diagnosis. Usually the patient complains of aural fullness, dysacusis and tinnitus and presentes effusion in the middle ear on physical examination.2,19 Such signs and symptoms are commonly attributed to otitis media with effusion secondary to Eustachian tube dysfunction and are mistakenly corroborated by the findings of conductive or mixed hearing loss on audiometry and type B tympanic curve on immittance. In this situation, it is only after the occurrence of clear, pulsatile and persistent otorrhea after ventilation tube placement for otitis media with effusion treatment that the diagnosis of CSF leak is suspected. This was the scenario that occurred with the reported patient. Other less frequent symptoms are headache, otalgia, dizziness and posterior (paradoxical) rhinorrhea.
However, in some patients, the initial clinical manifestation can be a complication such as meningitis, brain abscess and seizures. The risk of meningitis in patients with CSF leaks varies from 4% to 50%, depending on the fistula characteristics38 and the morbidity and mortality associated with this condition is significant, with a mortality rate as high as 30% in infections caused by pneumococcus.39 A recent survey has indicates an average of 19.6% of spontaneous temporal CSF leaks patients started clinical manifestation with meningitis and 4.8% of these patients had brain abscesses or seizures at some point during the evolution.5,10,17,19,21,24,26,28,29 Therefore, in adults with meningitis, especially those caused by pneumococcus, the possibility of spontaneous CSF leak should be considered and investigated.39 In prophylactic terms, prophylactic treatment with antibiotics has not demonstrated efficacy38 and the current recommendation is to carry out anti-pneumococcal, anti-meningococcal and anti-Haemophilus influenzae type b vaccination should also be considered, despite the lack of randomized clinical trials and case control studies that demonstrate a reduction in the incidence of meningitis in patients with CSF leaks who were vaccinated.40 These complications are what imposes the need for surgical treatment of spontaneous CSF leaks.2,11,14,19 However, specifically in relation to spontaneous temporal CSF leaks, no data were found on the impact of surgical approaches in reducing meningitis rates.
In exceptional situations, such as patients who refuse to undergo surgery or in cases which the anesthetic-surgical risk is high, it is reasonable to adopt an expectant approach, with careful surveillance and guidance on risks to patients, considering reports in the literature of group of patients with active CSF leaks which refused treatment or failed to repair and did not develop meningitis during an average observation period of 50.9 months.4 Thus, it is important to consider the diagnosis of spontaneous CSF leak in the hypotheses of adult patients who have a compatible clinical and with the referred alterations in complementary exams, especially if they have the predisposing conditions, in order to determine a correct diagnosis in a timely manner.
In relation to diagnosis, the role of high-resolution CT, allowing the defects location and the temporal bone anatomy study for adequate surgical programming, and the role of MRI, allowing the middle ear and mastoid contente differentiation (cerebrospinal fluid or meningoceles/meningoencephaloceles) is well established. Another information that can be shown by MRI is the empty sella, which is often present in cases of idiopathic intracranial hypertension, what may draw attention to this underlying diagnosis. Cisternography can also be used as a diagnostic resource in cases which the location of the bone defect was not clear by CT. Biochemical test such as the measurement of β2 transferrin in the otorrhea secretion can contribute to the diagnosis, since it deals with specific CSF protein. Despite the sensitivity of 99% and specificity of 97%, it has a considerable rate of false negatives, with detection ranging from 55 to 75% of cases, possibly due to small and intermittent drainage3,4,38 and impaired detection under infections or meningitis associated with Streptococcus pneumoniae.13 In addition, it has limited availability. Biochemical tests of glucose dosage (greater than 60% of serum glucose) and total proteins (greater than 200mg/ml) have poor sensitivity and specificity and, therefore, are not reliable.18
About the treatment of temporal CSF leaks, several surgical modalities are proposed. The most common are the transmastoid approach (TM), middle fossa craniotomy (MFC) or the combination of both techniques (TM + MFC). The choice depends on the location and size of the bone defect, the patient hearing thresholds and the surgeon experience. The use of the TM approach has its advantage in the relative technical facilty and minimal invasion compared to other modalities. It allows treatment of small (up to 1cm) and unique defects in the mastoid tegmen and posterior fossa plaque. However, the disadvantage of this modality is limitation of access to anterior tympanic tegmen defects, which would imply ossicular chain removal for manipulation of the area. In addition, when a major defect is found, this approach may not guarantee adequate space to obtain a successful osteodural defect repair.1
Some authors argue that the MFC approach is the best option for treating all types of temporal CSF leaks. The main advantage of this approach is an enlarged exposure of the entire middle fossa floor, allowing the anterior tympanic tegmen defects repair with ossicular chain preservation, being the surgical option widely indicated in normal hearing function patients, in addition to repairing the other regions of the tympanic and mastoid tegmens. In addition, it allows the correction of the superior semicircular canal dehiscence when it exists. However, it has its disadvantage in terms of the need to manipulate the temporal lobe, and therefore the risk of neurological complications and the need for admission to the intensive care unit and longer hospital stay.1,19,22
Other recent studies with promising preliminary results suggest the possibility of MFC approach with the aid of an endoscope, which could reduce neurological complications and surgical time.33,41 The TM + MFC approach combines the advantages of both techniques, allowing better management of large and multiple defects of the entire middle fossa floor length from a limited and focused subtemporal craniotomy ont the dehiscence, after the first site identification via mastoidectomy, which would tend to reduce neurological complications. However, it is associated with a longer operative time.3,22 In situations which there is no viable hearing or in case of high surgical anesthetic risk in a new surgical approach because of failure of the first repair, a subtotal petrosectomy can be performed.3,22
Regarding surgical effectiveness, the TM approach has been successful in 97.1% of cases and the MFC approach in 94.1%. This difference can be explained by the greater complexity of the cases typically handled by MFC. When performing combined procedures, the success rate has been 97.9%. Regarding complications, considering major complications (death, hearing loss, permanent functional deficits, meningitis, abscess, subdural hematoma, pneumoencephalus, stroke), the TM approach has presented a rate of 1.3%, the MFC approach of 9.1% and a TM + MFC approach of 1.9%.1 As for the repair of the osteodural defect, it is strongly recommended to perform multilayers repair, and different materials can be used. It is essential to cover the bone defect with rigid and long-lasting tissue capable of sustaining the intracranial content. Autologous materials such as bone and cartilage and synthetic materials such as silicone fasciae, stainless steel plates and titanium mesh are the most used. To reinforce the repair, with sealing purpose, autologous materials such as temporal fascia, muscle flaps, bone paté and fat and synthetic materials such as dural substitutes, biological glues, bone wax and hydroxyapatite cement can be used. It tends to give preference to autologous materials due to the lower chance of rejection and infection, lower cost, greater availability and less interference in ossicular chain mobility and in control image exams.1,3 In the reported patient, five layers of materials have been used for the repair, combined autologous and synthetic materials.
For the management of eventual encephaloceles or associated meningoencephaloceles, it is recommended to cauterize the nail with bipolar cautery and subsequent excision.22 The use of a lumbar drain during the intraoperative period is controversial as it may present additional postoperative risk, is associated with an increase hospital stay and does not contribute to an increase the success rates of repairs.19,22,41 A study have showed a 3% risk of minor complications and 5% of major complications. Potential complications include pneumocephalus, subdural or subarachnoid hemorrhage, meningitis, catheter retention, radiculopathy, headache and local wound infection.19 Another study have showed that the average hospital stay for TM approaches without lumbar drain was 1.6 days, while TM approaches with lumbar drain was 4.8 days and for MFC approaches, all with drain placement was 6.3 days.22 The lumbar drain is mainly used when dealing with MFC approach, as it allows the relaxation of the temporal lobe and minimizes the retraction, allowing the complete tegmen exploration to avoid multiple minor defects loss and to optimize major defects closure. It also allows decompression of the dural closure during the initial healing of the repair. In addition, it can contribute to the intracranial pressure verification, contributing to the diagnosis of idiopathic intracranial hypertension.42 When used, is recommended a drainage of 5-10ml/h and the maintenance for a maximum period of three days.22,41 Finally, the importance of diagnosis and adequate management of comorbidities associated with the development of spontaneous CSF leaks can not be neglected, as well the long-term follow-up of these patients, with regular intervals, because of the possibility of maintaining risk factors and development of osteodural fragility and dehiscence new areas.
Spontaneous temporal CSF leaks is a condition that require great clinical suspicion for investigation and, therefore, its knowledge is essential for the good clinical practice by otorhinolaryngologists, mostly it is a condition with increasing incidence in recent years and associated with serious complications if not properly managed.
None.
None.
The authors declare that there is no conflict of interest.

©2021 Moreira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.