Journal of
eISSN: 2379-6359


Case Report Volume 1 Issue 1
Head and Neck Surgery Department, Foundation Onkologikoa Fundazioa, Spain
Correspondence: Jose A Gonzalez Garcia, Head and Neck Surgery Department, Foundation Onkologikoa Fundazioa, Paseo Dr. Begiristain 121. 20018, Donostia-San Sebastian, Spain, Tel +34 943328127
Received: November 26, 2014 | Published: January 5, 2015
Citation: Garcia GJA. Misdiagnosis of a nasopharyngeal pleomorphic adenoma as a nasopharyngeal carcinoma. J Otolaryngol ENT Res. 2014;1(1):23-25. DOI: 10.15406/joentr.2015.01.00006
Pleomorphic adenoma is the most common benign tumor of the parotid gland, but may also arise from minor salivary glands. Salivary gland tumors arising out of the major salivary glands are rare and nasopharynx is an unusual site for pleomorphic adenoma tumor. Few cases have been presented in the literature. The preoperative diagnosis should link imaging tests, clinical and endoscopic examination and histological study; despite this, the use of intra operative diagnosis using the frozen section technique could lead to a misdiagnosis of nasopharyngeal carcinoma.
Keywords: pleomorphic adenoma, nasopharynx, transnasal endoscopic surgery, nasopharyngeal surgery, nasopharyngeal neoplasms
Salivary gland tumors include tumors arising from the major salivary glands (sublingual, submandibular and parotid glands) and tumors arising from the numerous minor salivary glands located in the upper aerodigestive tract. The Pleomorphic Adenoma (PA) usually appears on the parotid gland (80% of all PA cases) and represents 40-50% of all salivary gland neoplasms arising in the parotid gland. 20% of all PA arise in the rest of the major salivary gland or the minor salivary glands, but nasal or nasopharyngeal location in adults is quite uncommon.1‒3 The preoperative diagnosis of a nasal or a nasopharyngeal PA is very difficult, and imaging studies, nuclear medicine studies and preoperative and intraoperative biopsies may misdiagnosis a PA as a well differentiated nasopharyngeal carcinoma.
A 61-yar-old man was referred to our Head and Neck Surgery Department after completion of external radiotherapy treatment with the diagnosis of persistent nasopharyngeal T2N0 well differentiated carcinoma.
The patient referred right ear fullness and right nasal obstruction. Nasal endoscopy shows a rounded mass in the right wall of the nasopharynx with hyperkeratotic mucosa surrounding the mass. The tumor was affecting the right nasopharyngeal wall, the pharyngeal opening of the Eustachian tube, the posterior floor of the right nasal cavity and the soft palate (Figure 1).
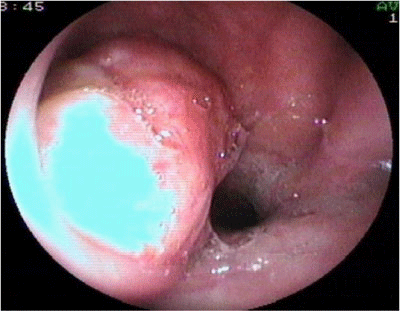
Figure 1 Endoscopic view through the right nasal cavity of a Pleomorphic Adenoma arising in the right lateral wall of the nasopharynx.
Computed Tomography demonstrated a right nasopharyngeal mass with slight contrasting uptake affecting the right nasopharynx without neck adenopathies (Figure 2).
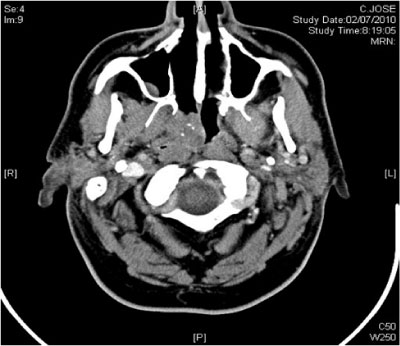
Figure 2 Axial CT scan showing a Pleomorphic Adenoma affecting the right choana and the right nasopharynx without clear radiologic malignancy.
PET-CT showed only a metabolic uptake in the right nasopharynx with a Standard Uptake Value of 5.5 (Figure 3).
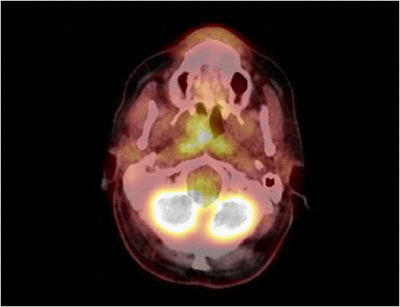
Figure 3 PET-CT scan showing only a metabolic uptake in the right nasopharynx due to Pleomorphic Adenoma.
TES was performed a few days later finding an encapsulated mass with seemingly a thick capsule, firmly adhered to the right wall of the nasopharynx. Intraoperative frozen sections of the tumor were informed as well differentiated squamous-cell carcinoma with basaloid areas and focal points of keratinization. Previous endoscopic ligation of the sphenopalatine artery was realized and the mass was extracted by the oropharynx and oral cavity due to its large size.
Right nasal cavity was packed and packing was removed in 48 hours and the patient was discharged without complications.
Final diagnosis: Cytopathologic study revealed a minor salivary gland tumor, with biphasic pattern and low prolipheration index with well defined borders and surrounded by a thick layer of fibrous tissue. Ki-67 prolipheration index <2%. Cytokeratin AE1/AE-3 was positive in the cellular areas and carcinoembryonic antigen (CEA) was negative demonstrating a PA with predominant cellular component (Figure 4). In this case multiple dilated thin-walled blood vessels filled with intraluminal deposits of eosin positive material were found leading to an intraoperative diagnosis of malignancy (Figure 5).

Figure 4 Microphotograph of a tumor showing a mixture of epithelial, myoepithelial and stromal components with normal minor salivary glands and a thick layer of fibrous tissue typical of minor salivary glands Pleomorphic Adenoma. Hematoxylin/eosin staining.
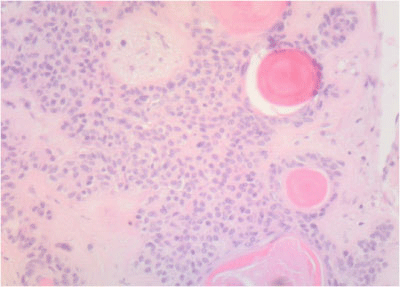
Figure 5 Intravascular deposits of cells and eosin positive myxoid material mimicking keratin nodules as described in some epidermoid carcinomas. Hematoxylin/eosin staining.
Four years after, the patient refers no symptom but xerostomy due to radiotherapy and presents a normal exploration of the right ear and the nasopharynx, with a scar in the inferolateral aspect of the right choana and normal movement of the nasopharyngeal opening of the Eustachian tube (Figure 6).
PA is a benign salivary gland tumor that exhibits a wide cytomorphologic and architectural diversity mixing epithelial cells, myoepithelial cells and a stromal and mesenchymal component. 20 % of all PA arise in the minor salivary glands located in the superior aerodigestive tract, most of them in the oral cavity, being the nasopharyngeal location extremely uncommon. The presenting symptoms vary from nasal obstruction to intermittent epistaxis, rhinorrhea, aural fullness and hypoacusis.4 PA should be differentially diagnosed from various other tumors, such as angiofibroma, harmatoma, epidermoid cyst, hemangioma, vascular malformations, nasopharyngeal carcinoma, lymphoma, non-epithelial tumors, etc.5
Image findings in PA vary from one tumor to other because of the heterogeneity of the histologic components and the numerous outer salivary glands locations. They are sometimes difficult to distinguish from low-grade malignancies or other benign neoplasms and clinical findings in exploration and endoscopy should support the image findings.6
The role of new imaging techniques as Magnetic Resonance spectroscopy or Positron Emission Tomography scan is still not well established and the use in this case was justified just because of the previous diagnosis of nasopharyngeal carcinoma.7
The treatment for newly diagnosed or recurrent PA is surgery, previously realized by lateral rhinotomy or other external approaches and now by endoscopic transnasal approach helped by trans-oral approach to avoid unnecessary morbidity. Transnasal endoscopic resection is not easy and complications as chronic otitis media with effusion, epistaxis, or local recurrence may occur. Radical resection with clear margins is mandatory avoiding recurrences and malignant transformation.8‒9 In this case, previous radiotherapy should lead to intraoperative bleeding, delayed scar formation, ulceration or necrosis with a subsequent bleeding risk and that is why an endoscopic sphenopalatine artery ligation was performed prior to excision of the tumor. Long term follow-up is necessary for early diagnosis of any sign of recurrence.4‒6
Misdiagnosis of PA is not uncommon, and it’s been well documented the diagnostic accuracy of fine needle aspiration and biopsies but intraoperative frozen sections of PA are not so well defined and more studies are needed to define the role of this technique in the diagnosis and management of PA as it´s not routinely used. Some kind of histologic findings in PA specimens could lead to a misdiagnosis of malignancy as intravascular tumor deposits like it was in this case,10 skeletal muscle differentiation11 or cutaneous adnexal differentiation and stromal metaplasia.12
None.
The authors declare that there is no conflicts of interest.

©2015 Garcia. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.