Journal of
eISSN: 2379-6359


Mini Review Volume 10 Issue 1
Zagazig University, Egypt
Correspondence: Ebtessam Nada, Zagazig University, Zagazig, Egypt, Tel +201227752699
Received: December 19, 2017 | Published: February 23, 2018
Citation: Nada E. Intra-operative monitoring during cochlear implant surgery. J Otolaryngol ENT Res. 2018;10(1): 00314 DOI: 10.15406/joentr.2018.10.00314
Introduction: Intra-operative monitoring during cochlear implant surgery is a tool to assess integrity of the equipment used and to assess the progress of array introduction and proper placement of the array with subsequent assessment of proper functioning of the device. Furthermore, Intra-op is used as a preliminary tool for subsequent CI mapping later on especially for difficult to test patients. Variable measures are present to perform Intra-operative measures include; electrophysiological measurement of electrode impedance (EI), electrically evoked compound action potential (ECAP), spread of excitation (SOE) and radiographic imaging modalities of plain X-ray radiograph, fluoroscopy, 3-dimensional (3-D) rotational x-ray, and intraoperative computed tomography (CT). Each of which has its own value and clinical implications.
Conclusion: Each of intra-operative measures is used each to assess a certain parameter. The collaboration of all measures together results in a successful anatomic and functional electrode placement to be ready for post-operative mapping.
Keywords: Cochlear implant, temporal bone surgery, electrode array, soft failure, hard failure
CI, Cochlear implant; OR, Operating room; EI, electrode impedance; ECAP, electrically evoked compound action potential; SOE, spread of excitation; CT, computed tomography; DSP, digital signal processing; RF, radiofrequency transmitter; PC, personal computer; IR, Intra-operative; ESRTs, Electrical stapedil reflex thresholds, ECoh G, Electrocochleography; EABR, Electric auditory brain response; SCC, Semicircular canal.
All CI systems have the following composition and functional units. An external unit "speech processor", consists of a digital signal processing (DSP) unit, a power amplifier, and an RF transmitter. The DSP is the brain of the CI system that receives sound, extracts features in the sound, and converts them into a stream of bits that can be transmitted by the RF link. The DSP also contains memory units or “maps” that store patient specific information. The maps and other speech processing parameters can be set or modified by a PC fitting program.1
The receiver-stimulator consists of a coil (RF receiver), a decoder, and a charge delivery system (stimulator). The receiver coil is in magnetic coupling with the transmitter coil and is located under the skin, picks up both the power and the data transmitted from the external coil. Data is decoded to produce the current wave form used to stimulate the auditory nerve via the electrodes located in the scala tympani.1
A feedback loop that monitors electric and neural activities in the implant and transmits the information back to the external unit (back telemetry) is also present. This allows the external unit to check status of the internal unit and to measure and monitor critical information regarding the electrode-tissue interface.2 These reverse telemetric capabilities are available in all current CI systems that have enabled the implanted device to send EI and ECAP information back to the speech processor and software for clinical interpretation.
Intra-operative monitoring during cochlear implant surgery is a tool to assess integrity of the equipment used, and to assess the progress of array introduction and proper placement of the array with subsequent assessment of proper functioning of the device. Furthermore, Intra-op is used as a preliminary tool for subsequent CI mapping later on especially for difficult to test patients.3
It is worth mentioning that optimal and atraumatic electrode insertion within the scala is an important pre-requisite for maximizing CI success. Furthermore, detection of faulty electrode insertion, or electrode malfunctioning or failed receiver-stimulator in the OR provides a chance for immediate intervention while the patient is still under anesthesia which lessens the incidence of revision.4
Intra-operative measures include; electrophysiological measurement of electrode impedance (EI), electrically evoked compound action potential (ECAP), and other objective measures used (electric auditory brainstem response 'EABR", and electric stapedial reflex thresholds "ESRT" and ECoh G used in hearing preservation surgery). Also, spread of excitation (SOE) and radiographic imaging modalities of plain X-ray radiograph, fluoroscopy, 3-dimensional (3-D) rotational x-ray, and intraoperative computed tomography (CT)(Figure 1).5–12
EI is a function of the array itself and the resistive characteristics of the fluid or tissue surrounding each electrode so, it provides information on electrode integrity, such as short or open circuits5 as well as information about the properties of electrode-tissue interface. Impedance in general refers to the resistance to the flow of energy through any medium. In CI electrode impedance refers to the measure of the opposition to electrical current flow across an electrode and thus, it is influenced by the electrode contact and the electrode lead that is coupled to the contact as well as by the medium surrounding the lead.6
Impedance measures are done to the array in package aiming at diagnosis of electrode array integrity before being used. Impedances in package are normally high (HI) and abnormality here is: presence of short circuits between any number of electrodes. After electrode insertion, open circuit is usually caused by faulty electrode contacts which will lead to a measurable impedance values at an infinity values indicating that larger amount of current is needed to measure impedances in this specified electrode or may be an indication of broken electrode leads. Testing multiple times or conditioning (passage of low current through the array) may improve high impedances in OR.7
On the other hand, a short circuit refers to establishment of relatively low resistance between 2 points in a circuit which typically are separated by a much higher electrical resistance. Ideally the term short means that 2 electrode leads /contacts are electrically coupled to one another in an unintended way so that a shorted electrode is identified as 2 electrodes that are electrically coupled and consequently possess an identical voltage that is distributed to each electrode when only one is stimulated and is caused by physical contact between 2 electrode contacts or electrode leads or by an electrical fault within the electrode lead/contact or excessive distortion or tension on the array.7
In contrast, the ECAP is an indicator of the neural responsiveness of the auditory nerve.8 ECAP recording is rapid, unaffected neither by motion artifact nor by type or depth of anesthesia, making it feasible in both the operating room and clinic.13,14 Intraoperative, ECAP measurements can be used to assess the response of a patient's auditory system to electrical stimulation immediately after proper intra-cochlear insertion of the CI electrode (Figure 2).8,15–17
ECAP is near field measured using intra-cochlear electrodes of the implant. Wave latencies of N1 and P1 are 0.2-0.4, 0.6-0.8 msec respectively. Amplitude: increases with increasing stimulus level, peak to peak amplitude can be as large as 1mV but usually it is hundreds of microvolt. It has the advantages of being measured in 95-96% of cases, relatively resistant to anesthesia and not contaminated by myogenic activity (muscle artifact) "because the response is measured using intra-cochlear electrode (not a surface/scalp electrode)", which in turn leads to lessening of averages required to record the response (10-100 sweeps) and hence, test time is short. ECAP is present in the first year of life and less affected by maturational factors.7
Previous research reported non-significant correlation between intraoperative ECAP values and postoperative speech performance.17 Although it is rare not to record ECAP intraoperatively; but if so, it doesn’t indicate absence of neither stimulation nor a dysfunctional device.
Another IO measure is EABR, in which a synchronous physiological response from the auditory nerve to structures in the brain stem is obtained in response to electrical stimuli applied through the implanted electrode array. Like the acoustic one, waves from I-V can be recorded but wave I and sometimes II can be obliterated by stimulus artifacts. In comparison with acoustic ABR, latencies are earlier about 1-2 m sec (direct stimulation of spiral ganglia).7
It is a far-field response from the auditory nerve up to the inferior colliculus. Wave V is larger than the acoustic one as electrical stimulation results in greater synchrony and broader spatial spread through the cochlea. EABR advantages are that it can confirm device function, auditory nerve function, adjuvant to processor programming, and verifying questionable behavioral responses, strong correlations are present between them at slow stimulation rates.8 But it can be contaminated by other physiological response such as those induced by electrical stimulation of facial or vestibular nerve.
EABR thresholds almost occur above behavioral thresholds and approximate or slightly exceeding upper comfortable levels and cannot be used alone to predict map levels, but it is helpful when limited behavioral responses are available.7
ESRTs, can confirm device function as well as response of the auditory system (to the level of the brainstem) to electrical stimulation and additionally, they have strong correlation with the C levels so, help in post-operative speech processing programming. But, being absent in a significant percentage of people and normal middle ear function is necessary for their recordings besides the need for additional equipment in the operating room (contralateral measurement) limit their use.7
Another intra-operative measure is SOE which has been reported as a useful intraoperative electrophysiological measure of CI electrode placement.9 SOE measurements provide information regarding the selectivity of neural excitation fields around each electrode; when these overlap, they can suggest presence of a tip rollover.18
On the other hand, intraoperative radiological imaging provides confirmation of proper, intra-cochlear placement of the electrode array.9,13,17,19,21 Plain radiographs are used to evaluate electrode location, position, and presence of tip rollover. In cases of abnormal cochlear anatomy, intraoperative fluoroscopy also has been advocated as a dynamic ‘‘real-time’’ assessment of CI electrode placement.11,13
Additionally, more sophisticated imaging modalities also have been used during CI surgery, including CT and 3-D rotational x-ray, but intraoperative access to these technologies is still uncommon (Figure 3).12,13
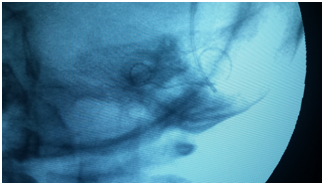
Intraoperative plain radiograph can identifies cases of improper electrode placement as placement in the superior SCC. Published literature contains 4 prior reports of electrode placement in the SCC in patients with normal cochlea-vestibular anatomy.20–23
In 2 of these 4 cases, intraoperative impedance and NRT showed measureable responses on all electrodes; radiologic confirmation was not performed. Postoperatively, vertigo with device activation prompted radiologic imaging with temporal bone CT with subsequent identification of incorrect electrode placement which required a second revision surgery for device removal and re-implantation.21–22 In the third case report, impedances indicated intact electrode circuitry, and 3 of 4 tested electrodes had measureable ECAP responses.20 In the last case report, no ECAP responses were obtained intra-operatively.23
Similarly, a report by Nevoux et al.24 described electrode placement in the intra-temporal carotid canal in a child with Connexin 26 mutation, normal cochlear anatomy, and a middle ear effusion at the time of implantation. Intraoperative monitoring consisted only of ECAP measurements, for which they report a complete lack of responses. Postoperative CT scan identified the extra-cochlear electrode location.
Lastly, intra-operatively, ‘‘hard’’ failure is characterized by a loss of telemetric ‘‘lock’’ or an inability of the external processor to communicate with the receiver-stimulator. Although lack of telemetric ‘‘lock’’ can relate to skin flap thickness, current surgical incisions have been evolved such that thinning of the flap over the device is no longer possible without extending or enlarging the post-auricular incision.
Intraoperative monitoring measurements can provide a valid basis for initial programming, especially in difficult-to-program populations such as very young children or those with multiple disabilities. Recent research in animal and human cadaveric models provide support for continuous impedance monitoring during electrode insertion as a method of assessing intra-cochlear physiology and preventing insertion trauma.25
Immediate intra-operative determination of device function abnormalities and proper electrode placement is important. IO monitoring, using EI, ECAP and plain film x-ray is feasible in routine CI surgery and may be normal in the majority of patients. The combination of the 3 modalities improves surgical outcome and reduces surgical revision rate because of hard failure of implant malfunction.
None.
None.

©2018 Nada. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.