Journal of
eISSN: 2379-6359


Case Report Volume 14 Issue 1
Department of the Rhinosinusology and Skull Base Section of the Otorhinolaryngology, Italian Hospital of Buenos Aires, Argentina
Correspondence: Carlos Santiago Ruggeri, Department of the Rhinosinusology and Skull Base Section of the Otorhinolaryngology, Italian Hospital of Buenos Aires, Perón 4190, Ciudad Autónoma de Buenos Aires, Argentina
Received: May 11, 2022 | Published: May 30, 2022
Citation: Zamar CE, Ragoni A, Ruggeri CS. Infraorbitary artery epistaxis. J Otolaryngol ENT Res. 2022;14(1):25-28 DOI: 10.15406/joentr.2022.14.00500
Epistaxis is a common complication of endoscopic sinus surgery. Bleeding occurs more frequently due to injury of the sphenopalatine artery branches. Pathologies of the maxillary sinus that produce bone resorption and descent from the orbital floor into the sinus, expose the orbital fat and the infraorbital artery (IOA) to an injury that can cause severe epistaxis which is difficult to treat. Two cases of epistaxis due to an injury of the infraorbital artery in the maxillary sinus are described in our study.
Keywords: infraorbitary artery, epistaxis, endoscopic surgery, maxillary sinus
Epistaxis is a frequent complication of endoscopic nasal surgery. The most common origin are the sphenopalatine artery branches. Other arteries such as the ethmoid or internal carotid can be injured during surgery and cause serious bleeding that can be life-threatening. Epistaxis originating from the infraorbital artery is rare and there are few reports in the literature. The artery can be injured in endoscopic surgery of the maxillary sinus or by facial trauma. Life-threatening traumatic epistaxis is approximately four times more likely to occur in LeFort type II and III and has been reported to be more common in high-energy trauma complicated with head trauma, cervical vertebral fracture, and other trunk trauma.1 Two cases of epistaxis produced from the IOA injury during endoscopic surgery of the maxillary sinus are described in this study. We consider that due to the infrequency, severity and difficult management of epistaxis originating in the AIO, the description of the following clinical cases is relevant.
Clinical cases
Case 1
24-year-old female patient.
She consulted due to swelling and anesthesia in the cheek of several years of evolution and mild left exophthalmos. Rhinoscopy and nasal endoscopy revealed a reduction in the left nasal cavity due to medial displacement of the medial wall of the maxillary sinus. Computed tomography (CT) revealed an expansive lesion in the left maxillary sinus with bone erosion of all its walls. The tumor was in contact with the inferior and lateral rectus muscles and extended to the pterygomaxillary fossa (Figure 1).

Figure 1 Coronal and axial CT: an expansive lesion is observed in the maxillary sinus with erosion of the bony walls and displacement of the eyeball.
Contrast-enhanced magnetic resonance imaging (MRI) revealed a hypointense tumor on T1 and hyperintense on T2 that displaced the eyeball upwards and contacted the inferior rectus muscle (Figure 2).

Figure 2 MRI: an expansive lesion is observed in the maxillary sinus with possible adherence to the inferior rectus muscle (arrows).
She had a history of 3 previous endoscopic surgeries for possible maxillary mucocele.
Surgical treatment was indicated. By an endonasal approach with endoscopes, the mucosa and medial capsule of the tumor were resected aspirating myxoid content (Figure 3).

Figure 3 Endonasal view with endoscopes.
A: aspiration through a middle antrostomy of myxoid material, B: middle mega-antrostomy after marsupialization and resection of a sector of the mucocele capsule for histopathological study. The arrow indicates the middle extrusion of the orbital fat.
When we did the marsupialization of the mucocele, at the roof of the maxillary sinus level, an extrusion of orbital fat and bleeding from the infraorbital artery occurred. By using bipolar coagulation, the bleeding could be resolved.
She remained hospitalized for 24 hours and had a good evolution. Histopathological diagnosis was fibrous tissue and chronic inflammation compatible with mucocele.
Case 2
Forty-two year old man.
He consulted for bilateral nasal obstruction of 1 year onset and mucous rhinorrhea.
Nasal endoscopy revealed polyps in the right nasal cavity and abundant mucous secretions and a septal deviation in the left nostril that blocked the passage of the endoscope.
A CT scan revealed a tumor that expanded the right maxillary sinus, eroded the medial wall of the maxillary sinus and displaced the nasal septum towards the contralateral nostril.
He also had bilateral ethmoid, sphenoid and frontal sinusitis.
The left maxillary sinus was hypoplastic and it was observed that the floor of the left orbit was lowered into the sinus (Figure 4).

Figure 4 Computed Tomography.
A-Coronal CT: an expansive lesion in the right maxillary and ethmoid sinus that displaced the nasal septum, and a descent of the left orbital floor is observed, B- Axial CT: a collapse of the left maxillary sinus walls is observed.
The MRI revealed a right expansive lesion with variable signal, predominantly hypointense on T2 and hyperintense on T1, the left maxillary sinus was hypoplastic and the floor of the eye descended into the sinus (Figure 5 ).
A lesion with variable signal is observed, which occupies the right sinus cavities, displaces the nasal septum and compresses the contralateral intersinus nasal wall causing a left maxillary sinusopathy, collapse of the sinus walls and descent of the orbital floor (the dotted line indicates the level of both orbital floors).The presumptive diagnosis was allergic fungal sinusitis.
He was started on oral corticosteroids 7 days before surgery (meprednisone 40mg/day in decreasing doses). A wide right middle antrostomy, anterior and posterior ethmoidectomy, sphenoidotomy and bilateral Draf II-b type frontal drainage were performed through an endonasal approach using endoscopes, resecting abundant allergic mucin. The nasal septum was perforated in its posterior sector.
The lateral wall of the left nostril was atelectatic and no anatomical reference such as the uncinate process or the middle turbinate could be identified. Using the inferior turbinate as a reference, the maxillary sinus was opened entering above the turbinate, and when attempting to enlarge the middle antrostomy, the orbital fat was exposed.
A prelacrimal approach was performed and the lower and middle antrostomy was completed (Figure 6).
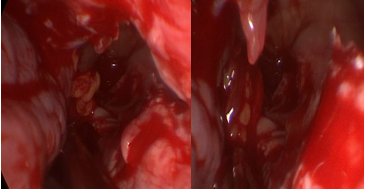
Figure 6 Endonasal view with 0º endoscopes.
Prelacrimal approach, lower and middle antrostomy through which the extruded orbital fat can be observed in the maxillary sinus (arrows).
At the end of the surgery, matrix haemostatic (Surgiflo®) was placed in both nasal cavities.
Seven hours later, he had an epistaxis at the hospital room and an anteroposterior packing with a double balloon (rapid-rhino®) was placed. Due to the persistence of bleeding, surgical revision was indicated.
By endonasal approach with endoscopes, an arterial bleeding from the roof of the left maxillary sinus was observed. It was very difficult to access and cauterize the IOA by the Prelacrimal approach, therefore an anterior maxillary sinusotomy was performed.
The IOA could be observed between the extruded fat within the maxillary sinus and was coagulated with bipolar (Figure 7).
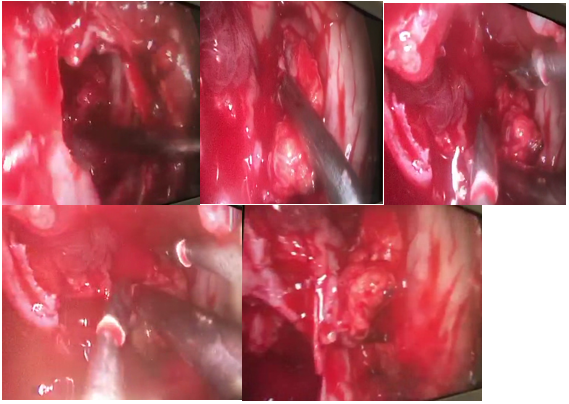
Figure 7 Endoscopic view through anterior maxillary sinusotomy.
A: maxillary anterior sinusotomy, B: dissection of the exposed fat to identify the IOA,
C-D: bipolar coagulation of the artery between the exposed fat, E: resolution of bleeding from the IOA, extruded fat is observed within the maxillary sinus (dotted arrow) and in the midline antrostomy (solid arrow).
.
He had a mild bleeding in the right nasal cavity, so the sphenopalatine artery was cauterized and bilateral anteroposterior packing with a double balloon was placed and removed 48 hours later.
The hematocrit dropped to 24, and there was fibrinogen and platelet deficiency.
It was interpreted as a consumption coagulopathy and he was transfused.
He did not have ophthalmological complications or facial anesthesia.
On the fourth day he was discharged from the hospital. Filamentous fungi without mucosal invasion were identified in the culture.
He had a good evolution.
The IOA arises from the internal maxillary artery in its pterygopalatine portion after the emergence of the posterosuperior alveolar branch and before the sphenopalatine artery. According to Traxler et al.,2 it can also originate in a common trunk with the posterior superior alveolar artery in 33% and its average caliber at its origin is 1.64mm (between 1.2 and 2.7mm). It then passes through the inferior orbital fissure and gets into the infraorbital canal along with the nerve to exit through the infraorbital foramen.
The IOA has a similar diameter to the sphenopalatine artery (1.0 +- 0.3mm /1.2 +- 0.2mm).3 The infraorbital canal may have anatomical variants which make it protrude more or less in the maxillary sinus. Type I does not protrude and type III descends into the maxillary sinus, type II is an intermediate variant.4 The largest protrusion exposes the artery to injury, especially when there is also a pathological descent of the orbital floor as can occur in the silent sinus or in other diseases such as mucoceles after the marsupialization.
There may also be dehiscence of the infraorbital canal. The IOA can be located medial, superior, inferior, or between the branches of the infraorbital nerve. No artery was located lateral to the infraorbital nerve. In a study carried out on anatomical specimens5 they found in 15 out of 20 a medial location of the artery in relation to the infraorbital nerve, which makes it more vulnerable to injury for a endoscopic surgery of the maxillary sinus. One of 20 sides (5%) had maxillary sinus hypoplasia, the authors found that this anatomical variant made it difficult to work within the sinus through the prelacrimal approach.
In the two cases described in our study, there was a descent of the orbital floor into the sinus and the AIO was located medial to the nerve. In the second case described, we had great difficulty to coagulate the AIO through the prelacrimal access and had to perform an anterior sinusotomy to aspirate, dissect and coagulate the artery with bipolar between the orbital fat. It is very rare to injure the infraorbital artery in endoscopic sinus surgery. In a PubMed search with the keywords epistaxis, infraorbitary artery, we found no reports of this complication.
In pathologies that produce osteolysis and displace the eye, such as mucoceles, when performing marsupialization and draining the mucoid content, the eyeball descends and the infraorbital artery may be exposed.
In our first case, the history of several previous surgeries possibly produced fibrosis and adherence of the mucocele capsule to the periorbita with a subsequent increased risk of arterial injury.
We also had to remove part of the capsule to obtain material for histopathological study and exclude an ameloblastoma. In the second case, the maxillary sinus was hypoplastic and atelectatic. When opening the ostium at the level of the middle meatus, orbital fat extruded, making it difficult to complete the middle maxillary antrostomy. We had to do a prelacrimal approach to perform a lower and middle antrostomy. We did not detect any arterial bleeding and we only placed hemostatic matrix in both nasal cavities at the end of the surgery.
The epistaxis occurred 7 hours later while the patient was in the room. This could be possibly attributed to episodes of coughing that may have traumatized the IOA exposed between the fat within the maxillary sinus. The resolution of the epistaxis was simple in the first case, due to the good exposure we had through the middle mega-antrostomy. In the second case the resolution of the epistaxis was very difficult due to the inadequate access we had through the left nostril, which was narrow and due to the extruded fat inside the sinus which prevented the visualization of the IOA. Direct access through anterior maxillary sinusotomy with the assistance of endoscopes allowed us to insert an aspirator and coagulate the artery with bipolar.
In both cases, surgical treatment of the epistaxis was performed. In the first, because the diagnosis was intraoperative and in the second because we did not suspect a hemorrhage from the IOA.
In traumas or when hemorrhage from the IOA is suspected, the embolization may be a better option since with this treatment there would be a lower risk of injury to the infraorbital nerve. The endovascular treatment is superior in identifying the bleeding point and selecting the blood vessel, and general anesthesia is not necessarily required.6 Complications of endovascular treatment for epistaxis and facial trauma include facial pain, facial paralysis, skin necrosis, palatal ulcer, diplopia, and cerebral infarction, regardless of the site of bleeding. However, the incidence of irreversible serious complications is low, ranging from < 2 to 17% of all complications. Conversely, the success rate of the procedure is high, with observed rates of 87-93%.7,8 Another surgical treatment option may be transoral paramaxillary cauterization with endoscopes of the internal maxillary artery.9 Packing would not be effective since the bleeding originates within the maxillary sinus
Epistaxis originating from the infraorbital artery is very rare and can be severe.
Bipolar coagulation of the artery through a wide middle antrostomy or an anterior maxillary sinusotomy allows the introduction of the endoscope, aspirator and bipolar to adequately locate the artery and cauterize it.
None.
The authors state that there is no conflict of interest.

©2022 Zamar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.