Journal of
eISSN: 2379-6359


Forum Article Volume 12 Issue 2
1Head of Department of Surgery, Mustansiriyah University, Iraq
2AL Yarmouk Teaching Hospital , D.MA. ST (High Diploma in Medical Audiology & Speech Therapy , Iraq
3Al-Yarmouk Teaching Hospital, Iraq
4Al-Jamhory Teaching Hospital, ENT Senior House officer, Iraq
Correspondence: Adnan Qahtan Khalaf, AL Yarmouk Teaching Hospital, ENT Specialist, Laser Surgeon Otolaryngologist, Iraq, Tel 009647901783546
Received: January 13, 2020 | Published: March 5, 2020
Citation: Yaseen ET, Qader AMA, Khalaf AQ, et al. Evaluation of graft taking and hearing threshold after tympanoplasty. J Otolaryngol ENT Re. 2020;12(1):37?44. DOI: 10.15406/joentr.2020.12.00453
Background: Tympanoplasty is one of the surgical procedures mainly aimed to restoring the hearing loss and eradication of chronic middle ear diseases.
Aim: To evaluate the hearing threshold preoperatively and postoperatively in patients who were subjected to tympanoplasty procedures.
Patients and methods: This prospective study included 27 patients attended ENT department of AL-Yarmouk Teaching Hospital ,from February -2017 to October- 2018 , The age of patients between (10-60 years) of both gender . they presented with hearing loss as a result of chronic suppurative otitis media. After full assessment and proper preparation , They underwent Tympanoplasty procedure through post auricular incision using underlay temporalis fascia graft , The type of Tympanoplasty procedure was planned according to the status of the middle ear and ossicular chain .To eradicate disease from both the mastoid and middle ear cavity procedure and could be combined with mastoidectomy, Patients were evaluated preoperatively and followed up for 6 months postoperatively, pure Tone Audiogram was done to asses the change in hearing.
Results: The study included 27 patients, (74.06%) of them were the age group between (21–40) years , the least number of patients were younger than 21 years and older than 51 years , 16 females (59.3%) and 11 males (40.7%) , Patients were classified according to tympanoplasty procedures into five groups :
(Group1):10 patients (37.03%) underwent only Type I tympanoplasty.
(Group2): 11 patients (40.7%) Type I with Cortical Mastoidectomy.
(Group3): 2 patients (7.4%) Type II with Cortical Mastoidectomy.
(Group4): 3 patients (11.1%) Type III with Cortical Mastoidectomy
(Group5): one patient (3.7%) Type III with Modified Radical Mastoidectomy. They had a mean Air-Bone gap improvements were (9.66 dB), (17.4 dB), (13.2 dB), (9.60 dB) and (5.54 dB) respectively. The overall graft success rate was (92.5%).
Conclusion: Significant improvement was noted in the subjective symptom of hearing loss following the tympanoplasty procedures. The mean Air-Bone gap closure was greatest for type I with cortical mastoidectomy; followed by type II with Cortical Mastoidectomy, type I alone and then type III with Cortical Mastoidectomy. Modified radical mastoidectomy was associated with the least hearing improvement as otherwise.
Chronic otitis media1
The diagnosis of chronic otitis media (COM) implies a permanent abnormality of the pars tensa or flaccida, most likely a result of earlier acute otitis media, negative middle ear pressure or otitis media with effusion. COM equates with the classic term chronic ‘suppurative’ otitis media (CSOM) that is no longer advocated as COM is not necessarily a result of ‘the gathering of pus’. However, the distinction remains between active COM, where there is inflammation and the production of pus, and inactive COM, where this is not the case though there is the potential for the ear to become active at some time. A third clinical entity is healed COM where there are permanent abnormalities of the pars tensa, but the ear does not have the propensity to become active because the pars tensa is intact and there are no significant retractions of the pars tensa or flaccida. ‘Healed COM’ can also be the end result of successful surgery.
COM classification |
Synonyms |
Otoscopic findings |
Healed COM |
Tympanosclerosis; |
Thinning and/or local or generalized opacification of the pars tensa without perforation |
Inactive (mucosal) COM |
Perforation |
Permanent perforation of the pars tensa but the middle ear mucosa is not inflamed |
Inactive (squamous) COM |
Retraction |
Retraction of the pars flaccida or pars tensa (usually posterosuperior) which has the |
Active (mucosal) COM |
|
Permanent defect of the pars tensa with an inflamed middle ear mucosa which |
Active (squamous) COM |
Cholesteatoma |
Retraction of the pars flaccida or tensa that has retained squamous epithelial debris |
Table 1 Classification of COM1
Inactive mucosal COM (dry perforation)
WHO includes inactive mucosal COM (dry perforation) under CSOM.
There is permanent perforation of the pars tensa but the middle ear and mastoid are not inflamed.
The mucocutaneous junction is usually located at the margin of perforation which can extend up to the fibrous annulus (Figure 1).
Active mucosal COM (perforation with otorrhoea CSOM1
There is chronic inflammation within the mucosa of the middle ear and mastoid with varying degrees of oedema, submucosal fibrosis, hypervascularity and an inflammatory infiltrate including lymphocytes, plasma cells and histiocytes. There is also an increase in the number of goblet cells and basal cell hyperplasia in the middle ear epithelium. Granulation tissue can occur and this is often clinically described as ‘aural polyps’ which have protruded through the perforated tympanic membrane. Some areas with active COM (both mucosal and cholesteatomatous subtypes) demonstrate focal areas of cholesterol granuloma formation, which microscopically consists of a giant cell reaction surrounding cholesterol clefts. Chronic inflammation can affect the whole of the middle ear cleft including the mastoid antrum and some surgeons believe it is important when a perforation is repaired that the whole of the infected mucosa and granulation tissue from the mastoid and middle ear space is removed in order to control the disease.
Active mucosal COM is often associated with destruction of the ossicular chain. The affected ossicles typically show areas of hyperaemia with proliferation of capillaries and prominent granulation tissue. The long process of the incus, stapes crura, body of incus and manubrium are involved in decreasing order of frequency (Figure 2).
Inactive squamous epithelial COM (retraction, atelectasis and epidermization)1
Negative static middle ear pressure can result in retraction (atelectasis) of the tympanic membrane. A‘retraction pocket’ consists of an invagination into the middle ear space of part of the tympanic membrane and this may be fixed, when it is adherent to the structures in the middle ear, or free, when it can move medially and laterally depending on the state of inflation of the middle ear “Epidermization” is a more advanced type of retraction and refers to replacement of the middle ear mucosa by keratinizing squamous epithelium without retention of keratin debris (Figure 3).
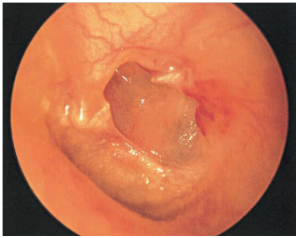
Figure 3 Posterior atelectactic pars tensa with erosion of posterior canal wall, as well as long process of incus and stapes superstructure.
Active squamous epithelial COM (acquired cholesteatoma)1
Cholesteatoma is a benign keratinizing epitheliallined cystic structure found in the middle ear and mastoid. It can cause destruction of the local structures ossicular chain and otic capsule, thereby leading to complications such as hearing loss, vestibular dysfunction, facial paralysis and intracranial disease or infection. The term “cholesteatoma “ was first coined by the German physiologist Johannes Muller in 1838 (Figure 4).
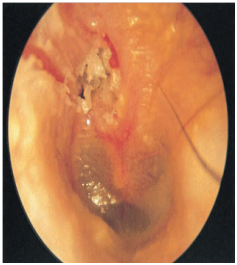
Figure 4 Active squamous COM clearly visible affecting pars flaccida in the attic. The white debris is squamous epithelial debris, indicative of cholesteatoma.
Pathophysiology of tympanic membrane perforation2
The transmission of sound through damaged middle ears changed by way of several mechanisms.
A hole in the tympanic membrane will cause
Depending on their positions; reduce the mechanical coupling between the remaining intact portion of the tympanic membrane and the malleus.
Tympanoplasty: is a surgical procedure performed to eradicate infection and restore the function of the middle ear.
Wullstein introduced a classification for tympanoplasty based on two things:
Wullstein’s original classification scheme breaks tympanoplasty into five subtypes:
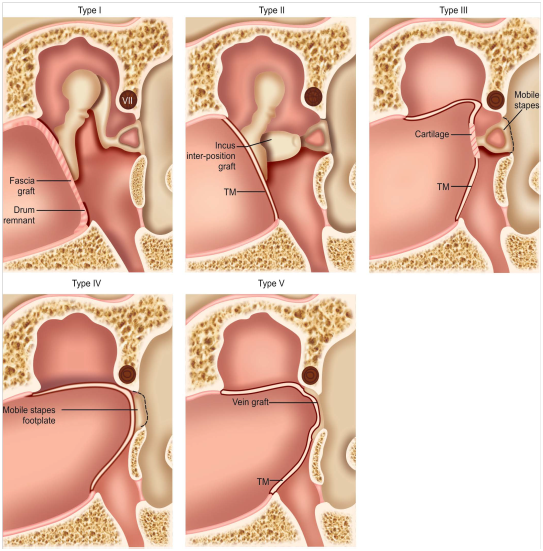
Figure 5 Type I–V tympanoplasty.4
Indications of tympanoplasty3
Contraindications3
Patients and methods
Preoperative preparation: included routine blood tests, Chest X- ray, ECG, Echogardiogram (in indicated patients) as a part of requirements for fitness for general anesthesia.
Preoperative audiometry: had been done within one week preoperatively for assessment of conductive hearing loss and Air-Bone gap.
Tympanometry
Operative preparations: Councelling of the patients about the surgical procedures and their possible benefits and complications. Informed consent of patients and their relatives had been taken.
Vital parameters: Temperature, Pulse rate, blood pressure and respiratory rate were recorded.
Skin preparation: shaving the hair of the post auricular region 3cm inside the hair line. Position of the patient -supine with the face turned to opposite side. Sterilizing the surgical field had been done by povidone iodine.
Covering the patient by surgical towels.
Examination under microscope: was done to complete the assessment of the external auditory canal, perforated tympanic membrane, ossicular bones status and the condition of middle ear mucosa. Tympanic membrane was visualized. Re-freshening of the perforated margins using suitable surgical instrument was done.
Post auricular incision done.
Gentle forward traction was applied to the pinna by assistant. A curved incision was made 5 mm behind the post auricular fold started at 12 o’clock position superiorly (at the root of zygoma) and terminated at 6 o’clock
Position below (Figures 1).
The subcutaneous tissue and muscles of auricle were divided with a knife or diathermy needle using a cutting current.
Harvesting of the temporalis fascia graft had been done, (Figures 6&7).
Temporalis fascia graft harvesting procedures:
Surgical procedures
Type of Tympanoplasty procedure was planned according to the status of the middle ear and ossicular chain.
To eradicate the disease from both the mastoid and the middle ear cavity tympanoplasty could be combined with mastoidectomy (Figure 9). For all above procedures post aural approach with William Wildespost auricular incision were used. All patients underwent tympanoplasty. TM grafting was done using temporalis fascia employing underlay technique.
Type I tympanoplasty: Done for patients with CSOM of inactive mucosal variety. It simply involves reconstruction of tympanic membrane with middle ear exploration to ensure normality (ossicular chain intact and mobile).
Type I tymapnoplasty with cortical mastoidectomy: Was done in patients with persistant Otorrhoea via post aural approach, the mastoid cortex was drilled, the antrum and the accessible mastoid air cells were cleared of the disease. Patency of the aditus and antrum was checked and established. The edges of the TM perforation were freshened, tympanomeatal flap was elevated, and mesotympanum was visualized. Ossicles were inspected for signs of erosion and adhesions present between the ossicles and the mucosa were cleared using sickle knife to determine mobility of ossicular chain .TM grafting was done by underlay technique using temporalis fascia graft, small bits of gel foam were placed in the middle ear. Tympanomeatal flap was repositioned. External auditory canal was packed with medicated gelfoam and gauze pack. Post aural incision was closed in layers using 3-0 vicryl. Post aural dressing was done and mastoid bandage was applied.
Type II tympanoplasty with cortical mastoidectomy: At the time of exploration of middle ear there was intact incus and stapes with erosion of malleus. TMF graft placed onto incus or onto malleus remnant also it could be restore the function of lever mechanism by placing an inter position graft between long process of incus and stapes head, there was somewhat discharge in the middle ear and wet mucosa so that cortical mastoidectomy had been done concomitantly.
Type III tympanoplasty with cortical mastoidectomy: The surgical approach to the middle ear is same above. After inspecting the middle ear, depending upon the type of ossicular destruction type III tympanoplasty was done, for conservation of hearing. The ossicular defect was erosion of the lenticular process of Incus and destruction of the Stapes superstructure was seen. Cortical mastoidectomy was a part of surgery according to condition of the middle ear.
Type III tympanoplasty with modified radical mastoidectomy: with the same approach as described previously, the mastoid antrum and air cell system, Aditus, Antrum, attic and middle ear are converted into a single cavity. The facial bridge removed and the facial ridge lowered, thus exteriorizing the cavity. Tympanoplasty was carried out at the same sitting. in this patient the disease was limited to the epitympanum is simply exteriorized by removing portions of the adjacent superior and posterior canal wall and the cholesteatoma matrix on the lateral surface excised and eroded incus was replaced by interposition of a piece of conchal cartilage.
Postoperative care
Day 0: observation of patient about vital signs, assessment of facial nerve function and started IV antibiotics (third generation cephalosporin: Cefitriaxone 1 gram/24hours) and analgesia.
Day 1: change of dressing, weber test was done, assessment of facial nerve function, same medications as above.
Day 2: change of dressing, weber test was done, assessment of facial nerve function, continue dose of antibiotics and analgesia.
Day 3: change of dressing, Removal of corrugate drain, assessment of facial nerve function, same medications as above.
Day 4: change of dressing, same medications as above.
Day 5: change of dressing, same medications as above.
Day 6: change of dressing, same medications as above.
Day 7: removal of stiches of post auricular incision, continue dose of antibiotics.
Day 14: removal of the wick and examine the ear under microscope.
Postoperative audiometry done at sixth week, third and sixth months
The Patients were counseling about the procedure before audiometry and adequate time was taken for every test. We tried that the Pure Tone Audiogram to be done on the same audiometer and by the same technician for all patients who were included in the this study, (Figure 10).
Statistical analysis: All data analyze dusing SPSS (version 23) computer program. Statistical analysis included descriptive statistics like: a tables and figures, Chi-Square test for qualitative variables. Independence t- test was used to compare between mean. In this analysis, the statistical significant association was determined. All P values were based on 2-sided tests, and P < 0.05 was considered statistically significant.
Twenty seven patients with CSOM were included in the study, 74.06% of them were the age group between 21 –40years, the least age groups were less than 21 years and above 51years (Table 1). The mean age of patients was (25.4 years). Mean duration of perforation was 10±8 years. The studied patients had hearing loss as preoperative symptom, 23 patients had discharge and 9 patients had tinnitus, (Table 2). Patients distribution according to the site of perforation of tympanic membrane were as following: 9 patients (33.3%) anterior perforation, 7 patients (25.9%) posterior perforation and 11 patients (40.7) subtotal perforation , As shown in (Figures 11). According to air-bone gap classifications, no significant differences were observed between patients with air-bone categories regarding age, gender and perforation site (p>0.05), (Table 3).
Age group |
NO. |
% |
10 - 20 |
2 |
7.40 |
21 - 30 |
13 |
48.14 |
31 - 40 |
7 |
25.92 |
41 - 50 |
4 |
14.81 |
51 - 60 |
1 |
3.70 |
Table 2 Distribution of patients according to age
Symptoms |
Patients NO. |
% |
Hearing loss |
27 |
100.0 |
Ear discharge |
23 |
85.1 |
Tinnitus |
9 |
33.3 |
Vertigo |
7 |
25.9 |
Otalgia |
3 |
11.1 |
Table 3 Clinical characteristics of patients preoperatively
Operative findings: In the operative theater and during the examination of patients under microscope patients were classified into five groups as shown in (Table 4).
Group |
Surgery type |
NO |
% |
1 |
Type I |
10 |
37.0% |
2 |
Type I+CM |
11 |
40.7% |
3 |
Type II+CM |
2 |
7.4% |
4 |
Type III +CM |
3 |
11.1% |
5 |
Type III + MRM |
1 |
3.7% |
Table 4 Types of Tympanoplasty (total number of patients, n = 27)
These groups are as following
Group 1: 10 patients (37.03%) presented with inactive mucosal CSOM with no involvement of ossicular chain and dry healthy middle ear mucosa, the patients had been undergone Type I Tympanoplasty alone.
Group 2: 11 patients (40.7%) presented with active mucosal CSOM, normal ossicullar chain and wet middle ear mucosa, the patients had been undergone Type I Tympanoplasty with CM.
Group 3: Two patients (7.4%) presented with active mucosal CSOM , erosion of malleus normal incus and stapes and incudostapedial joint , also there was somewhat wetness in mucosa of middle ear, the patients had been undergone Type II Tympanoplasty with CM.
Group 4: Three patients (11.1%) presented with active mucosal CSOM , erosion of mallus, incus and distortion of incudostapedial joint normal , continuous discharging middle ear , the patients had been undergone Type III Tympanoplasty with CM.
Group 5: One patient (3.7%) presented with sequamous CSOM presented as attic cholesteatoma. patient had been undergone Type III Tympanoplasty with MRM.
The overall graft success rate is (92.5%) and distribution of success and failure rates for each Tympanoplasty type shown in (Table 5). With the 15th patients (55.5%) showed good hearing improvement with gain of more than 20 dB , 3 patients (11.1%) showed no improvement (<5 dB); while a hearing improvement between 5 dB to 10 dB was seen in one patient (3.7%) , hearing improvement between 11 dB to 20 dB was seen in 8 patients ( 29.6%).
Procedures |
Graft success |
Graft Failure |
Type I (n=11) |
10 (90.9%) |
1 (9.1%) |
Type I +CM (n=11) |
11 (100%) |
- |
Type II +CM (n=2) |
2 (100%) |
- |
Type III +CM (n=4) |
3 (75%) |
1 (25%) |
Type III +MRM (n=1) |
1(100%) |
- |
Table 5 Success and failure rates for tympanoplasty procedures
Tympanoplasty is a reconstructive operation of tympanic membrane performed to prevent recurrent ear discharge and to improve hearing loss caused by tympanic membrane perforation.4 The patients with CSOM were found to be highest in the age group between )21 – 30( years , (48.14% ( which is consistent with that study of Loy et al.,5 This is also agree with kaur et al.,6 who found the commonest age group between( 20-29)years. The records high frequency of CSOM in females (59.2%) which is consistent to study done by Webb and Chang,7 they had reported a higher prevalence of COM in females in comparison to males patients. The site of perforation had no effect on hearing gain (p=0.9), this agree with Karela M,8 Stated that the site of perforation were noted as playing no particular role in hearing improvement. In contrast to the current study many authors as Carr and Uyar,9,10 believe that the perforations location play more important role in surgery success and hearing gain , Also Vartiainen E, Nuutinen J,11 stated that Perforations in the anterior quadrant of the TM represent a worse surgical access in order to reach the anterior border and they are also less vascularized owing to which they are considered an important success factor for surgery, Hallik et al.,12 in his long term results of tympanoplasty found that the anterior perforations healed more poorly.
Its was found that 19th patients (70.3%) showed A-B gap within 20 dB after six months post operatively. Only 8 patients (29.6%) had A-B gap persisting over 20 dB. This is agreed with Gersdorff M,13 who mentioned that Postoperative hearing outcomes were considered successful, if the postoperative air-bone gap was within 20 dB. The success rates vary in the literature, from 75 to 98%. It is noteworthy that Costa SS,14 mentioned that multiple surgeons (including residents) performed the various procedures which could have led to differing results based on their surgical skills. It should be noted that these surgeries are complicated procedures and their success also depends on quality of post-operative care. patients in Group (2) Type I with cortical mastoidectomy showed the best mean of hearing improvement of (17.4 dB), followed by Group(3) type II with CM which had a mean of hearing improvement of (13.2dB). Patient in Group(5) Type III combined with modified radical mastoidectomy showed a very poor mean hearing improvement (5.54 dB).
In contrast to the study Gersdorff M,13 stated that the correlation in the improvement gained in the various types of tympanoplasty, maximum improvement was seen in patients undergoing type I with cortical mastoidectomy. Patients having no improvement were seen in all types of tympanoplasty, signifying that many factors are responsible for the success of the surgical procedure. In Group (1) Type I tympanoplasty alone and Group (4) type III with CM showed an equal mean hearing improvement with (9.6 dB) for each group whereas for Group (2) the results were better. This is agree with Uyar Y,15 who mentioned that tympanoplasty associated with CM increase hearing improvement postoperatively and in contrast to this, Bhat KV,16 who stated that there were no statistically significant differences in hearing improvement.
According to comparison between Group (1) Type I tympanoplasty alone and Group (2) Type I tympanoplasty accompanied by CM:
The hearing improvement in Group (2) Type I Tympanoplasty with cortical mastoidectomy (17.4 dB) was better than that for Group (1) Type I Tympanoplasty without cortical mastoidectomy (9.66dB).
This disagree with Krishnan,17 who membered that Mastoidectomy did not seem to play a significant beneficial role as regards the postoperative hearing gain.
Also in contrast to the study, Albu et al.,18 found that cortical mastoidectomy offers no additional benefit regarding hearing gain over myringoplasty.
In the current study, the graft success rate was (100%) for Group (2) Type I Tympanoplasty with CM whereas it was (90.9%) for Group (1) Type I Tympanoplasty alone.
This is disagree with Mishiro et al.,19 who recorded graft success rate was better for myringoplasty alone in comparison to myringoplasty with CM , the success rate was (93.3%) of patients underwent myringoplasty without cortical mastoidectomy and in 90.7% of patients with myringoplasty and cortical mastoidectomy. Mishiro et al.,19 conducted a retrospective analysis of 251 ears with non-cholesteatomatous chronic otitis media They concluded that mastoidectomy is not helpful in tympanoplasty for non-cholesteatomatous chronic otitis media and reported an air-bone gap of 0-20 dB in 81.6% of patients underwent tympanoplasty with cortical mastoidectomy and 90.4% for patients underwent tympanoplasty without cortical mastoidectomy.
In contrast to the current results, Balyan et al.,20 presented a retrospective analysis of 323 patients with non-cholesteatomatous chronic otitis media. They separated the cases into three groups 1 (n=53) consisted of cases of chronic suppurative otitis media treated by tympanoplasty without mastoidectomy. Group 2 (n=28) included cases of chronic suppurative otitis media treated by tympanoplasty with mastoidectomy. The ears in both these groups were discharging severely at the time of surgery. Group 3 (n=242) included patients whose ears were dry at the time of surgery but who had previous recurrent episodes of suppuration and were treated by tympanoplasty without mastoidectomy. They observed that tympanoplasty without mastoidectomy is the preferable treatment.
Agreement with the results , a study done by McGrew et al.,21 examined the effect of mastoidectomy with canal wall up ( Group A) on 484 , post infectious, unoperated, non - cholesteatomatous TM perforations v/s myringoplasty alone (Group B) and showed graft up take was 93% in group A 91.6% in group B Also, agreement with the study by Sonkhya et al.,22 reported that aerating mastoidectomy is beneficial in patients with chronic otitis media as it restores the connection between the middle ear and mastoid and creates a physiological buffer. 212 patients of granulomatous chronic otitis media were managed by tympanoplasty with aerating mastoidectomy using modified intact canal wall technique and were followed for a minimum period of 12 months. Also agreement by Eliades and Limb,23 who conducted a study on 26 patients to review the surgical outcomes for patients with perforations resulting from CSOM without choleasteatoma and concluded that there was no additional benefit to performing mastoidectomy with tympanoplasty for uncomplicated tympanic membrane perforations. Patients with more complicated disease benefitted from adition of a mastoidectomy.
Significant improvement was noted in the symptom of hearing loss following the tympanoplasty procedures for CSOM without cholesteatoma. The mean Air-Bone gap closure is greatest for type I with cortical mastoidectomy; followed by type II with CM, type I alone and then type III with CM. Modified radical mastoidectomy was associated with the least hearing improvement as otherwise.
None.
There are no conflicts of interest.
None.

©2020 Yaseen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.