Journal of
eISSN: 2379-6359


Research Article Volume 16 Issue 1
1Department of Pediatric Otorhinolaryngology, University Hospital Brno and Faculty of Medicine, Masaryk University, Czech Republic
2Global Medical Affairs, Czech Republic
3GSK Consumer Healthcare SARL, A Haleon company, Switzerland
4GI&D OTC, Haleon, USA
Correspondence: Ivo Slapak, MD, PhD, Department of Pediatric Otorhinolaryngology, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Černopolní 9, 61300 Brno, Czech Republic, Tel 00420532234225
Received: February 25, 2024 | Published: March 4, 2024
Citation: Slapak I, Novak P, Hagen M, et al. Effectiveness of intranasal saline cleansing methods for removal of particulate matter. J Otolaryngol ENT Res. 2024;16(1):15-22. DOI: 10.15406/joentr.2024.16.00541
Purpose: High concentrations of inhalable particulate matter (PM, aerodynamic diameter 2.5–10 µm) are associated with increased risks of respiratory diseases, cardiovascular diseases, and adverse pregnancy outcomes. PM can disrupt the nasal epithelial barrier, leading to vulnerability to respiratory disease. Nasal saline washing can help support nasal functioning by removing trapped PM. We aimed to determine which nasal saline administration technique provided the best intranasal saline deposition and to assess the effectiveness of intranasal saline cleansing solutions for removing PM-simulating dust.
Methods: We conducted 3 in vitro studies using a nasal cast coated with an artificial mucus. Study 1 evaluated the deposition patterns of 3 nasal sprays administered with different techniques. A lateral image was taken after each administration to quantify the exposure area. Studies 2 and 3, in which PM-simulating dust was added to the nasal cast, evaluated the effectiveness of 4 intranasal saline sprays administered with the line-of-sight (LoS) method (head tilted sideways 45°, spray angle 0° from vertical) for washing away PM-simulating dust. The percentage of PM removed was quantified from pre- and post-washing images and from a high-accuracy liquid particle counter analysis of cast run-out.
Results: Study 1 demonstrated that the LoS method provided the best intranasal saline deposition. Studies 2 and 3 showed that intranasal saline administration with this method effectively recovered and removed the PM-simulating dust from the mucus-coated cast.
Conclusion: These results support the benefit of nasal saline washing with LoS administration, suggesting that this method should be recommended for nasal spray use to effectively remove PM. Future investigations are warranted to explore the benefits of nasal washing in a variety of clinical settings. Nasal saline cleansing can help preserve and maintain normal nasal functioning, possibly with long-term effects of helping to reduce the impact of air pollution on health.
Keywords: air pollution, nasal mucosa, nasal sprays, saline solution, particulate matter, respiratory system
WHO, World Health Organization; PM, particulate matter; LoS, line-of-sight; 3D, 3-dimensional; HIAC, high accuracy liquid particle counter; SD, standard deviation; CV, coefficient of variation; a, alpha; CI, confidence interval; N/A, not applicable
Epidemiological data indicate that air pollution is the largest environmental risk factor for disease and mortality worldwide.1,2 World Health Organization (WHO) data indicate that 99% of the global population breathes air that contains high concentrations of pollutants and that is not compliant with the limits stated in the WHO Global Air Quality Guidelines.1,3 The combined effects of outdoor air pollution (generated by cars, power plants, wood and agricultural burning, and other industrial processes) and household air pollution (generated by burning of biomass for heating and cooking, cigarette smoke, and household cleaners) are associated with 7 million premature deaths each year, making air pollution a major public health issue.1,3 The impact of air pollution is not limited to underdeveloped countries or densely populated urban centers but extends to developed countries and rural areas as well.4,5
Inhalable particulate matter (PM), categorized as PM10, consists of dust, pollen, mold, and other solid and liquid particulates with aerodynamic particle sizes between 2.5 and 10 µm.6 Fine PM, categorized as PM2.5, is derived from sources of pollution found both outdoors6 and indoors.7 Generally, small particles ranging from approximately 0.5 µm to 5.0 µm are deposited in the respiratory bronchioles, and those particles ≤1 µm can reach the alveoli, causing potential toxic effects.7,8 Size barriers to PM in the nasal cavity are reported to range from 11.0 to 7.0 µm in the nasal passages and from 7.0 to 4.7 µm in the pharynx.8 PM>3 µm are mainly deposited in the anterior part of the nose, which can then be mechanically removed by sneezing or blowing the nose.9
Exposure to PM increases the levels of inflammatory cytokines and inflammatory cell counts in the nasal mucosa.10-12 High PM levels are positively associated with increased risks of respiratory diseases (e.g., asthma, chronic obstructive pulmonary disease, lung cancer), cardiovascular diseases (e.g., cerebrovascular disease, ischemic heart disease), and adverse pregnancy outcomes.13,14 Cleaning the nasal mucosa with a saline solution is recommended to help clear PM to mitigate adverse effects on the body in infants, children, and adults.15 The established benefits of nasal saline washing have been previously described to result from thinning of the mucus, improving mucociliary clearance, decreasing edema, and reducing inflammatory mediators in the nasal and sinus cavities.15,16 This is especially important in infants, where nasal blockage can interfere with feeding and sleeping.15 In children and adults, adjuvant treatment with nasal saline irrigation is recommended for use in viral upper respiratory tract infections, allergic rhinitis, and sinusitis,15,17,18 and improvements in nasal mucociliary clearance were observed after nasal saline washing in individuals with acute and chronic sinusitis and allergic rhinitis.16,19 However, there remains a need to investigate the effects of nasal washing on reducing exposure to airborne pollutants and on promoting the natural defense physiology of the nasal epithelium. It is possible that by helping to remove trapped PM from the nose, nasal saline washing may reduce the effects of air pollutant exposure and support healthy nasal functioning.15
Nasal spray application technique is important for effective delivery,20 as optimal nasal deposition pattern influences effective cleansing of the nose (e.g., washing away debris, inflammatory mediators, and trapped airborne particles)15 and nasal mucociliary clearance.16,20 We reviewed the literature to determine the optimal method of nasal spray administration and found that consensus is lacking; methods vary according to factors such as head position, volume and frequency of administration, spray angle, and compliance. Expert advisors recommended the line-of-sight (LoS) method of administration (head tilted sideways 45°, spray angle 0° from vertical) as a preferred method; thus, we conducted 3 in vitro studies that used a 3-dimensional (3D)-molded nasal cast replicating the human nasal cavity to provide information on the optimal technique for administration of nasal cleansing solutions and the effectiveness of nasal cleansing solutions for the removal of PM.
All studies were carried out at Next Breath LLC, a division of AptarGroup Inc., Baltimore, MD, with the Transparent Nasal Cavity model LM-005 (Koken Co. Ltd., Tokyo, Japan) nasal cast, which measured 10.5 (L) x 9 (W) x 9 (H) cm and was coated with an artificial mucus. The methods of each study are summarized briefly below and illustrated in Figure 1. More detailed descriptions are provided in the Supplementary Materials.

aFor 1 product, only 1 sample was evaluated (1-second spray duration). LoS, line of sight.
bOtrivin Breathe Clean Nasal Spray and Rinazina Aquamarina were administered with a 3-second spray duration. The Physiomer nasal spray was administered with a 1-second spray duration. Physiomer delivers more volume per in mL/sec than Otrivin Breathe Clean Nasal Spray and Rinazina Aquamarina. To achieve comparable volumes per product, the actuation time of Physiomer was reduced.
Figure 1 ThreeOverview of the design of Studies 1–3. (a) sagittal and frontal views of the nasal cast. (b) cast positions for nasal sprays analyzed in Study 1. (c) methods of Studies 2 and 3.
Study 1: Nasal cast deposition study
This study characterized and quantified the total area of the deposition pattern of 3 nasal cleansing solutions to the nasal cavity and potential deposition into the nasopharynx region. The solutions contained aloe vera, seawater, and purified water (Otrivin Natural Plus Nasal Spray), benzododecinium bromide, polysorbate 80, 0.9% sodium chloride, 90% ethanol , glycerol, and purified water (ProRhinel Nasal Spray), and 0.65% sodium chloride, purified water, disodium and monosodium phosphate, benzyl alcohol, and benzalkonium chloride (Ocean Saline Nasal Spray); all were from Haleon, formerly GSK Consumer Healthcare, Warren, NJ).
Each nasal spray unit was inserted approximately 1/2 inch (~1.27 cm) into the nostril, per the package insert. The angle and orientation of the nasal cast for the assessment of each product are shown in Figure 1b. All units were actuated manually, and the nasal cast orientation was maintained for approximately 10 to 20 seconds after each actuation. The artificial mucus changed color from white to pink when exposed to the water contained in the spray (Figure 2).
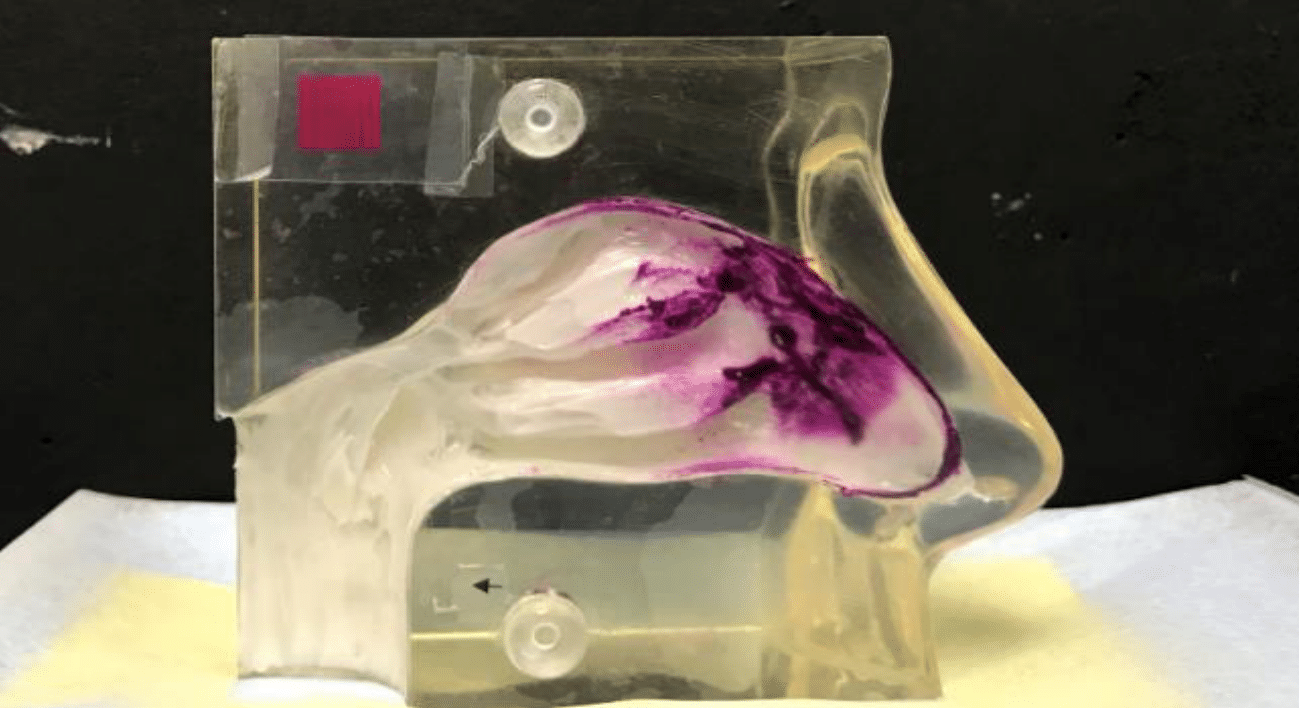
Figure 2 Representative image of nasal spray deposition in the nasal cast model.a
aThe model corresponds to the size of an adult nose.
Analyses were carried out after 1 and 2 sprays. A lateral image was captured after each sample collection, and photo analysis software installed on NextBreath Computer (Next Breath LLC, a division of AptarGroup Inc., Baltimore, MD) was used to quantify the total area of exposure. All pink regions were quantified as part of the deposition area (entire nasopharynx region in addition to the nasal cavity).
Studies 2 and 3: Nasal cast cleansing studies
Study 2 was a pilot study to assess the removal of Urban Dust PM (NIST Urban Dust Particulate Matter provided by Next Breath LLC, a division of AptarGroup Inc., Baltimore, MD) from a nasal cast with 4 nasal spray products (Haleon, formerly GSK Consumer Healthcare). The products contained aloe vera, seawater, and purified water (Otrivin Natural Aloe Vera Nasal Spray), aloe vera, seawater and purified water (Rinazina Aquamarina Delicata), benzododecinium bromide, polysorbate 80, 0.9% sodium chloride, 90% ethanol, glycerol, and purified water (ProRhinel Nasal Spray), and 0.74% w/v sodium chloride, glycerin, monosodium phosphate dihydrate, disodium hydrogen phosphate, disodium EDTA, and purified water (Otrivin Breathe Clean Nasal Spray). Study 3 was a follow-up study to quantify the percentage of Urban Dust PM removed by 3 nasal spray products (Haleon, formerly GSK Consumer Healthcare)—Otrivin Breathe Clean Nasal Spray, Rinazina Aquamarina, and a 100% seawater nasal spray (Physiomer) and was powered to show statistical significance.
In both studies, Urban Dust PM was introduced into the mucus-coated nasal cast cavity with a nasal powder insufflator (Dry Powder Nasal Insufflator provided by Next Breath LLC, a division of AptarGroup Inc., Baltimore, MD; Figure 3a). Spray deposition in the turbinate region was achieved with the LoS method of administration (nasal cast tilted sideways 45°, spray angle 0° from vertical; Figure 3b); each unit was actuated manually to rinse off the PM.
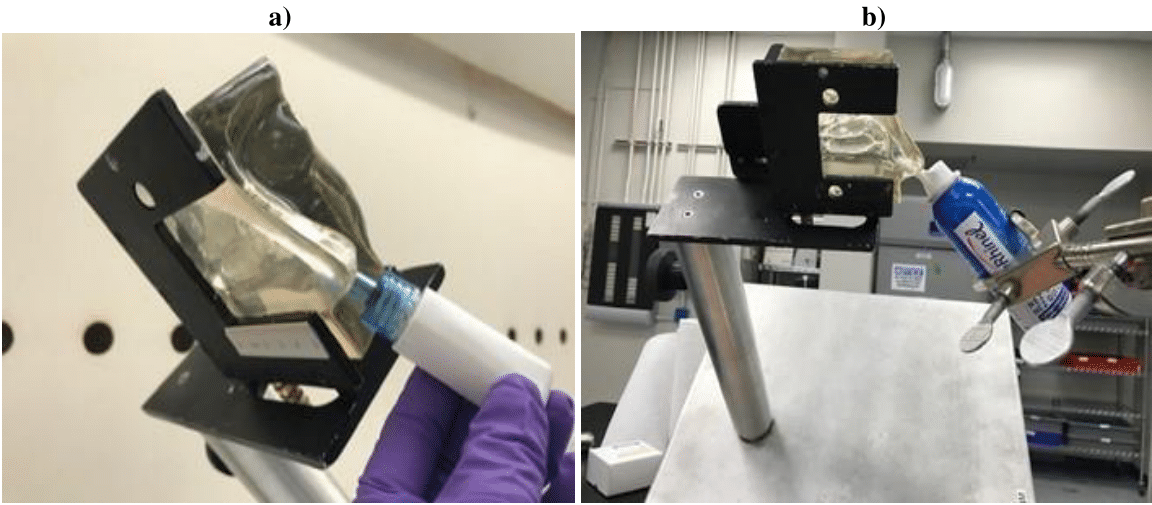
Figure 3 Devices used with nasal cast model. (a) nasal cast with dry powder insufflator. (b) nasal cast with bag on valve application.
Images of the nasal cast were taken before and after rinsing with each of the nasal sprays, and the percentage of PM particles removed was determined with Adobe Photoshop CS5 software. The number of particles removed was also quantified with a high accuracy liquid particle counter (HIAC); samples were collected from the back of the cast (nasopharynx region) run-out and from the entire cast and sent to Gateway Analytical (Gibsonia, PA) for particle count analysis, along with a representative Urban Dust PM sample.
Statistical analyses
Descriptive statistics were used to summarize the deposition area after 1 and 2 sprays of each product in Study 1 (mean, standard deviation [SD], coefficient of variation [CV]) and to summarize the PM recovery and removal with each spray in Study 2 and Study 3 (mean, geometric mean, SD, CV, alpha [a], 95% confidence interval [CI]).
Study 1: Nasal cast deposition study
With Otrivin Natural Plus Nasal Spray, overall spray weights ranged from 61.62 to 77.01 mg, and the spray covered the nasal valve and leading edges of the inferior, middle, and superior turbinate. Coverage was increased with 2 sprays versus 1 (Table 1), with an overall average deposition of 11.0 cm2 versus 8.1 cm2, respectively. No deposition occurred in the nasopharynx region, and no dripping occurred in the nasopharynx, philtrum, or lip regions.
|
Spray area (cm²) |
||
|
|
1 spray |
2 sprays |
|
Otrivin Natural Plus Nasal Spray |
||
|
Overall average |
8.64 |
11.01 |
|
Overall SD |
1.21 |
1.91 |
|
%CV |
14 |
17.3 |
|
ProRhinel Nasal Spray |
||
|
Overall average |
11.1 |
18.39 |
|
Overall SD |
1.54 |
1.91 |
|
%CV |
13.9 |
10.4 |
|
Ocean Saline Nasal Spray |
||
|
Overall average |
10.23 |
14.25 |
|
Overall SD |
1.41 |
3.69 |
|
%CV |
13.7 |
25.9 |
Table 1 Overall deposition area of nasal saline sprays
CV, coefficient of variation; SD, standard deviation
With ProRhinel Nasal Spray, overall spray weights ranged from 267.10 to 578.69 mg, and the spray covered the rear areas of the nasal valve and the inferior, middle, and superior turbinate. Application of 2 sprays versus 1 covered the deeper areas of the middle turbinate based on visual assessment, and overall average deposition was 18.4 cm2 versus 11.1 cm2, respectively. This spray provided less coverage in the front portion of the nasal valve than Otrivin Natural Plus owing to the position of the nasal actuator in the nasal valve. Some dripping was observed in the nasopharynx, but not in the philtrum or lip regions.
With Ocean Saline Nasal Spray, overall spray weights ranged from 71.05 to 136.82 mg, and the spray covered the nasal valve, the inferior and middle turbinate, and the leading edges of the superior turbinate. Application of 2 sprays versus 1 covered the deeper areas of all 3 turbinates, and overall average deposition was 14.3 cm2 versus 10.2 cm2, respectively. This spray provided more coverage in the front portion of the nasal valve compared with Otrivin Natural Plus and ProRhinel. No dripping was observed in the nasopharynx, philtrum, or lip regions.
The deposition patterns of these formulations are summarized qualitatively in Table 2. Findings of this study suggested that the LoS administration method (used with ProRhinel Nasal Spray) provided the best deposition (Figure 4), with the largest overall average deposition area after 1 and 2 sprays, the highest spray weights, and coverage of all 3 turbinates.
|
Product |
Deposition |
|
Otrivin Natural Plus Nasal Spray (nasal pump bottle) |
1° deposition on leading edge of turbinates and nasal valve |
|
ProRhinel Nasal Spray (bag-on-valve) |
1° posterior deposition between middle and inferior turbinates |
|
Ocean Saline Nasal Spray (squeeze bottle) |
1° deposition in nasal valve and cavity floor |
Table 2 Qualitative summary of nasal cast deposition of nasal saline sprays (study 1)
Studies 2 and 3: Nasal cast cleansing studies
Based on the results of Study 1, the LoS administration method was used in Studies 2 and 3 to provide the most effective means of washing off deposited PM from the nasal cast. Figure 5 shows a representative example of a nasal cast before (a) and after (b) PM washing with Otrivin Breathe Clean Nasal Spray. The percent recovery and removal of deposited PM with each formulation analyzed are discussed below and summarized in Tables 3 & 4, respectively (Study 2), and in Tables 5 & 6, respectively (Study 3).
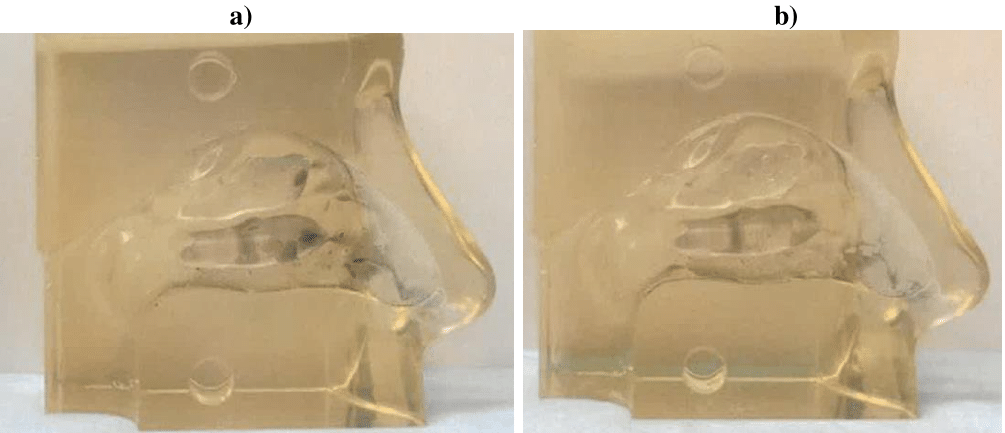
Figure 5 Nasal saline washing of a nasal cast coated with PM Urban Dust in Study 3. (a) before nasal washing with Otrivin Breathe Clean Nasal Spray. (b) after nasal washing with Otrivin Breathe Clean Nasal Spray administered in the LoS direction.
|
|
Percent total recovery from replicate samples |
|||
|
|
Otrivin Natural Aloe Vera |
Rinazina Aquamarina Delicata |
ProRhinel |
Otrivin Breathe Clean |
|
Mean |
89% |
86% |
87% |
87% |
|
Geometric mean |
89% |
86% |
87% |
87% |
|
SD |
5.90% |
6.70% |
6.00% |
6.50% |
|
%CV |
6.6 |
7.8 |
6.9 |
7.4 |
|
Alpha (a) |
0.05 |
0.05 |
0.05 |
0.05 |
|
SD (s) |
0.06 |
0.07 |
0.06 |
0.06 |
|
Sample size |
9 |
9 |
9 |
9 |
|
95% CI |
||||
|
Lower limit |
85% |
82% |
83% |
83% |
|
Upper limit |
93% |
91% |
91% |
92% |
Table 3 Summary of PM recovery in study 2
CI, confidence interval; CV, coefficient of variation; PM, particulate matter; SD, standard deviation.
|
|
HIAC analysis |
|
|
|
|
|
|
Otrivin Natural Aloe Vera |
Rinazina Aquamarina Delicata |
|
ProRhinel |
Otrivin Breathe Clean |
|
Mean |
31.14% |
32.62% |
|
32.59% |
29.51% |
|
Geometric mean |
29.03% |
30.42% |
|
28.41% |
27.43% |
|
SD |
10.90% |
11.40% |
|
15.50% |
11.90% |
|
%CV |
34.9 |
35 |
|
47.5 |
40.5 |
|
Alpha (a) |
0.05 |
0.05 |
|
0.05 |
0.05 |
|
SD (s) |
0.11 |
0.11 |
|
0.15 |
0.12 |
|
Sample size |
9 |
9 |
|
9 |
9 |
|
95% CI |
|
||||
|
Lower limit |
24% |
25% |
|
22% |
22% |
|
Upper limit |
38% |
40% |
|
43% |
37% |
|
|
Adobe Photoshop image analysis |
||||
|
Mean |
49% |
43% |
|
46% |
41% |
|
Geometric mean |
49% |
42% |
|
46% |
41% |
|
SD |
6.60% |
6.80% |
|
2.20% |
4.10% |
|
%CV |
13.4 |
15.7 |
|
4.8 |
9.8 |
|
Alpha (a) |
0.05 |
0.05 |
|
0.05 |
0.05 |
|
SD (s) |
6.6 |
6.8 |
|
2.2 |
4.1 |
|
Sample size |
9 |
9 |
|
9 |
9 |
|
95% CI |
|
||||
|
Lower limit |
45% |
39% |
|
45% |
39% |
|
Upper limit |
53% |
47% |
|
48% |
44% |
Table 4 Percent PM removal in study 2
CI, confidence interval; CV, coefficient of variation; HIAC, high accuracy liquid particle counter; PM, particulate matter; SD, standard deviation.
|
|
Otrivin Breathe Clean |
Rinazina Aquamarina |
|
Mean |
83% |
99% |
|
Geometric mean |
82% |
99% |
|
SD |
1.60% |
2.50% |
|
%CV |
1.9 |
2.5 |
|
Alpha (a) |
0.05 |
0.05 |
|
Sample size |
3 |
3 |
|
95% CI |
||
|
Lower limit |
81% |
96% |
|
Upper limit |
84% |
101% |
Table 5 Summary of PM recovery in study 3
CI, confidence interval; CV, coefficient of variation; PM, particulate matter; SD, standard deviation.
|
|
HIAC analysis |
|
|
Otrivin Breathe Clean |
Rinazina Aquamarina |
|
|
Mean |
52.65% |
57.56% |
|
Geometric mean |
52.30% |
56.35% |
|
SD |
7.60% |
13.80% |
|
%CV |
14.5 |
23.9 |
|
Alpha (a) |
0.05 |
0.05 |
|
Sample size |
9 |
9 |
|
95% CI |
||
|
Lower limit |
48% |
49% |
|
Upper limit |
58% |
67% |
|
Adobe Photoshop image analysis |
||
|
Mean |
39% |
58% |
|
Geometric mean |
32% |
57% |
|
SD |
22.70% |
12.60% |
|
%CV |
58.5 |
21.6 |
|
Alpha (a) |
0.05 |
0.05 |
|
Sample size |
3 |
3 |
|
95% CI |
||
|
Lower limit |
13 |
44 |
|
Upper limit |
64 |
73 |
Table 6 Percent PM removal in study 3
CI, confidence interval; CV, coefficient of variation; HIAC, high accuracy liquid particle counter; N/A, not
applicable; PM, particulate matter; SD, standard deviation.
The percent recovery of PM was consistent with all 4 products in 9 replicate runs from the nasal cast in Study 2 and with Otrivin Breathe Clean and Rinazina Aquamarina in 3 replicate runs from the nasal cast in Study 3. In both studies, the accuracy of the insufflation and rinse methods was demonstrated by the ≥80% recovery of deposited PM removed from the nasal cast for each product.
The geometric mean removal of Urban Dust PM is shown for Otrivin Natural Aloe Vera, Rinazina Aquamarina Delicata, ProRhinel, and Otrivin Breathe Clean in Study 2 in Table 4 and for Otrivin Breathe Clean and Rinazina Aquamarina in Study 3 in Table 6. As measured by HIAC, the geometric mean removal of Urban Dust PM was similar among the products evaluated in Study 2 (29%, 30%, 28%, and 27% for Otrivin Natural Aloe Vera, Rinazina Aquamarina Delicata, ProRhinel, and Otrivin Breathe Clean, respectively, Table 4) and among the products evaluated in Study 3 (52% and 56% for Otrivin Breathe Clean and Rinazina Aquamarina, respectively, Table 6). As measured by Adobe Photoshop image analysis, the geometric mean removal of Urban Dust PM was similar among the products evaluated in Study 2 (49%, 42%, 46%, and 41% for Otrivin Natural Aloe Vera, Rinazina Aquamarina Delicata, ProRhinel, and Otrivin Breathe Clean, respectively, Table 4), while in Study 3, that for Otrivin Breathe Clean was 32% and that for Rinazina Aquamarina was 57% (Table 6).
In Study 3, HIAC analysis was more robust, repeatable, and accurate for quantification than Adobe Photoshop image analysis, while in Study 2, the opposite was true (possibly due to subjectivity inherent in the Adobe Photoshop analysis method). However, as Study 2 was a pilot study, it was not designed to demonstrate statistical significance.
Air pollution from indoor and outdoor environments results in exposure of the nasal epithelium to PM, which has the potential to induce a variety of deleterious effects on the respiratory and cardiovascular systems.13,14 The toxicity of PM deposition in the nasal epithelium involves inflammation and oxidative stress that lead to a loss of barrier function.10–12 We hypothesized that use of intranasal saline cleansers may aid in removing PM from the nose to promote better health, potentially helping to modify the PM-associated increased risks of adverse effects. We carried out 3 nasal cast studies to identify the optimal technique for nasal saline administration and to assess the effectiveness of nasal cleansing solutions for PM removal.
Nasal casts are useful tools for evaluating the deposition of nasal delivery systems.21 Use of a nasal cast in the studies described here provided valuable information for the development of an effective nasal saline washing method. The results of Study 1 demonstrated the differences in nasal spray deposition area obtained with a variety of application methods and identified the LoS method as the approach that provided the best overall deposition. This finding addresses a gap in the literature regarding the optimal method of nasal saline administration and is supported by unpublished expert recommendations. The results of Studies 2 and 3 demonstrated that administration of these intranasal saline solutions with the LoS method effectively washed away PM-simulating dust that had been applied to the nasal cast.
The influence of application technique on efficiency was shown in Study 1. While heavy coverage in the nasal valve and front portion of the turbinate regions was observed with Otrivin Natural Plus Nasal Spray and Ocean Saline Nasal Spray, among the products analyzed, ProRhinel Nasal Spray, applied with the LoS method, provided the most coverage, with heavy coverage in the turbinate regions of the nasal cast (possibly due to the increased spray weight, and differences in spray geometry and pattern provided by the nozzle). In the pilot study carried out to assess the effectiveness of this approach for nasal washing (Study 2), the results showed that LoS application of nasal saline sprays washed away 41–49% of Urban Dust PM deposited in the nasal cast. The results of the follow-up study (Study 3) demonstrated that LoS application washed away 53–58% of the PM deposited in the nasal cast.
These findings are supported by the results of previous studies showing the importance of application angle for nasal spray deposition and demonstrating the clinical benefits of nasal saline washing.15,16,19,22–26 A study that evaluated the use of nasal casts constructed by 3D printing based on information from individual patient computed tomography scans found that deposition of cromolyn sodium spray in the turbinate region using a 30° angle of administration was enhanced by using patient-specific angles that accounted for individual patient characteristics.22 A study using a 3D in vitro cell model of human airway epithelium (MucilAirTM-HF) exposed to pollutants (PM2.5-like diesel particulate matter and PM10-like fine dust) showed that mechanical saline washing with Otrivin Natural Aloe Vera (Haleon [formerly GSK Consumer Healthcare]) improved measures of PM-induced deregulation of human nasal epithelial cells including restoring mucociliary clearance, maintaining tissue integrity, and lowering inflammatory mediators.27 In patients with sinusitis or rhinitis, regular use of nasal saline cleansing has been shown to improve nasal symptoms15,23–26 and mucociliary clearance.16,19 In children with rhinitis from cold or flu, adding regular saline washing to standard medication enabled faster resolution of symptoms, and continuing regular saline washing after the acute illness resulted in a reduced incidence of upper respiratory tract infections (URTI).26 In another study, patients with sinonasal disease who used nasal saline irrigation for treatment showed improvements over 6 weeks in severity and duration of numerous symptoms, such as nasal congestion and discharge, as well as improvements in nasal cleanliness and health status.25 Among adults with acute URTI, a higher proportion of those who regularly used nasal irrigation had ≥30% symptom score reduction from baseline in nasal congestion and runny nose compared with those who did not use nasal irrigation.23 Mucociliary clearance times were significantly shortened after regular nasal saline washing in studies of individuals with rhinitis and sinusitis,16,19 consistent with a benefit of removal of debris.
The in vitro studies described here have limitations. The main limitation is their in vitro nature, as use of a nasal cast does not allow for assessments of human factors, including mucociliary clearance,21 adverse events, and compliance. In addition, nasal casts can only approximate the geometry of the nasal cavity, which varies considerably among individuals of different ages, genders, and ethnicities.21,22 Other limitations of our studies include the differences among the nasal sprays evaluated relative to their physical and chemical properties and volumes delivered.20
Our findings suggest that nasal saline cleansing with the LoS method of application seems to be the optimal method to remove PM from the nasal cavity, and findings in the published literature support the hypothesis that nasal saline cleansing can improve mucociliary activity to help remove PM from the nose. Together, these effects may lead to cleaner breathing. Future clinical investigations are warranted to explore the extent of these potential benefits in a variety of settings. It is hoped that adoption of nasal saline cleansing for routine nasal hygiene to help maintain normal nasal functioning may have a role in reducing the effects of PM and ultimately lead to a reduction in the impact of air pollution on health.
The LoS method of nasal saline spray administration achieved optimal intranasal deposition and effectively removed trapped PM from a nasal cast, supporting the benefit of regular nasal saline washing with LoS administration to help remove trapped PM from the nose.
We would like to acknowledge NextBreath, LLC, a division of AptarGroup Inc., Baltimore, MD, for their contributions in conducting the tests, analyzing the data, and supporting the study design, as well as the key contributions of Nicolas Le Rat, Project Warsaw R&D lead.
Supervision, Design, Methodology, and Managing the studies with NextBreath for Study 2 and Study 3 Nasal Cast Cleansing Studies: AM
Data analysis and interpretation: IS, PN, MH, AM, MFP
Review and Approval for Reports for Study 2 and Study 3 Nasal Cast Cleansing Studies: AM
Manuscript preparation: original draft preparation, review, and editing: IS, PN, MH, AM, MFP
All authors have read and approved the final manuscript for publication.
Funding for this study was provided by Haleon (formerly GSK Consumer Healthcare). All listed authors meet the criteria for authorship set forth by the ICMJE.
The data that support the findings of this study are available from the corresponding author, IS, upon reasonable request.
IS declares that he has no financial conflicts related to this manuscript. PN, MH, MFP, and AM are Haleon employees.
Materials and methods
Study 1: Nasal cast deposition study
All testing was performed at room temperature in a humidity-controlled room at a relative humidity <45%. To visualize spray deposition, we coated the vestibules, nasal cavity, and nasopharynx of the cast with equal amounts (~7 g total) of Sar-Gel® (Arkema, King of Prussia, PA, USA), which changes color from white to pink when exposed to water. Samples were collected from the beginning-of-unit life (BOL), middle-of-unit life (MOL), and end-of-unit life (EOL).
Studies 2 and 3: Nasal cast cleansing studies
Deposition of each nasal spray product in the turbinate region of the nasal cast was achieved by fixing each product on a support, with the nozzle of the sample aimed towards the center of the eye pupil of the nasal cast in the line-of-sight (LoS) direction. Each product was sprayed manually into the nasal cavity to rinse off the Urban Dust particulate matter (PM).
For quantification with Adobe Photoshop imagery software of the percentage of Urban Dust PM particles removed, images of the nasal cast were taken in 3 vertical segments of the nasal cavity after the particles were introduced into the cast and after the deposited particles were rinsed from the cast with each of the nasal sprays.
For quantification with high accuracy liquid particle counter (HIAC) of the number of particles removed, sets of samples were collected from the back of the nasal cast (nasopharynx region) run-out and from the entire nasal cast. Sterile water was used as a final rinse of the nasal cast during sample collections. 15 mL of 1 M NaOH was introduced into the collected samples to dissipate the artificial mucus from the Urban Dust PM. Each sample was shaken for 3 minutes with a vortex shaker. The samples were collected with a filtration process with a metal filter. Each dried filter with sample was diluted with sterile water (30 mL) and sonicated for 30 seconds. The metal filters loaded with Urban Dust PM samples were allowed to dry for 60 minutes. The collected samples were sent to Gateway Analytical for particle count analysis by HIAC. In addition, a representative PM sample was sent to Gateway Analytical for particle count using HIAC. The PM was diluted to 500 mL in particle-free water, sonicated for 2 minutes, and gently inverted and mixed prior to particle count. Particle count was detected for PM size categories >2 to >100 mm. The total percent PM recovery was measured for each run by determining the total amount of PM deposited from the back (run-out) and from the entire cast (full rinse) over the total amount of PM introduced into the nasal cast.

©2024 Slapak, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.