Journal of
eISSN: 2379-6359


Research Article Volume 13 Issue 2
1Head of the Department, Department of Conservative Dentistry and Endodontics, AB Shetty Memorial institute of Dental Sciences, Nitte deemed to be university, India
2Post Graduate Student, Department of Conservative Dentistry and Endodontics, AB Shetty Memorial institute of Dental Sciences, Nitte deemed to be university, India
Correspondence: Prof. Dr. Mithra N Hegde, Head of the Department, Department of Conservative Dentistry and Endodontics, AB Shetty Memorial institute of Dental Sciences, Nitte deemed to be university, Mangaluru, Karnataka, India, Tel 9845284411
Received: February 27, 2019 | Published: March 9, 2021
Citation: Hegde MN, Mrinalini. Effect of probiotics on total salivary antioxidant level and physical properties of saliva: An ex-vivo study. J Otolaryngol ENT Res. 2021;13(2):13-16. DOI: 10.15406/joentr.2021.13.00483
Background: Role of oxidative stress in pathophysiology of various diseases has already been established. Antioxidants act as free radical scavenger and prevent damage to cell, at the same time affects the metabolism of several pathogenic microbes. With increasing incidence of antibiotic resistance, alternative therapeutic treatment options are coming into picture. WHO claims probiotics to be the next most important immune defence system following antibiotic resistance. Even though there are several studies emphasising on the positive outcome of probiotics on diseases, their exact mechanism of action still remains debatable. The present study tried to unveil the antioxidant property of probiotics in saliva along with its effect on salivary pH and buffering capacity.
Methods: Unstimulated saliva from 10 individuals were analysed for total antioxidant level, pH and buffering capacity using spectrophotometer, pH paper and buffering strips respectively, before and after probiotic intake for 2 weeks.
Results: Probiotic intake led to increase in total salivary antioxidant level with no significant changes in pH and buffering capacity. Results obtained were statistically analysed using “paired t-test”.
Conclusions: Probiotics are effective in increasing antioxidant level thereby minimizing cellular damage and hence can be used for prevention of several diseases. Also increase in antioxidant level can be easily assessed in saliva, thus, saliva can be used as a routine diagnostic tool.
Keywords: probiotics, saliva as biomarker, salivary antioxidant level, salivary pH, buffering capacity of saliva
Oxidative stress is a homeostatic event that arises due to imbalance in pro-oxidant and antioxidant level in the cell.1 Such disparity results in generation of free radicals leading to lipid peroxidation, protein denaturation, DNA hydroxylation and ultimately causes apoptosis of the cell.2 Excessive amount of these reactive radicals compromises the cell viability and contributes to several diseases including the ones associated with oral cavity like dental caries, periodontitis and oral precancerous lesions.3 With wide emergence of disease over the past few years and the knowledge of oxidative stress being a major determinant in their pathophysiology has shifted our preventive and therapeutic focus towards antioxidant modality.4 One such innovative approach is bacteriotherapy using probiotics. Probiotics refer to live non-pathogenic microorganisms which when administered in adequate amounts, confer health benefits.5 This good old concept of bacteriotherapy has been known for its beneficial effect and are said to be helpful in reducing oxidative stress, thereby minimizing the damage.6 However, exact mechanism is yet to be deciphered. Evidence has showed that probiotic bacteria exhibit significant antioxidant abilities both in in-vitro as well as in-vivo.7-10 However, the clinical studies performed, evaluated the antioxidant property of probiotics in plasma.
Saliva being an alternative diagnostic tool poses several advantages over plasma. Its availability, easy collection, possibility of repeated non-invasive sampling, simpler technique and faster interpretation makes it ideal for screening, diagnosing and monitoring various diseases.11 Very few studies have been performed on understanding the pathophysiology of oxidative stress in saliva and the potential role of probiotics on salivary antioxidant level have never been evaluated. Also the effect of probiotics on salivary buffering capacity and pH is not well understood. Thus, the aim of the study was to evaluate the effect of probiotics on total salivary antioxidant level and physical properties of saliva (pH and buffering capacity).
Armamentarium used:
Subject recruitment
After obtaining the institutional ethical clearance (ABSM/EC06/2019), study was conducted in the month of January over a period of 2 weeks. Only subjects who had given voluntary informed consent were included in the study.
Inclusion and Exclusion criteria were developed.
Inclusion criteria:
Exclusion criteria:
Saliva collection
On the day of saliva collection, subjects were instructed not to eat or drink anything for at least 1 hour prior to the collection. To control circadian variations, samples were collected between 9-10 a.m.
Methodology
Saliva was allowed to accumulate in the floor of the mouth and subjects were instructed to collect it into sterile saliva vial (Passive drool method). Samples were transported to the laboratory within 30 minutes of collection. Salivary analysis was done for pH, buffering capacity and total antioxidant level within 1 hour.
|
Sample No. |
Buffering capacity |
pH |
Total antioxidant capacity In Mm/L |
|||
|
Before |
After |
Before |
After |
Before |
After |
|
|
1 |
4 |
4 |
7 |
7.2 |
1.283 |
1.257 |
|
2 |
4 |
3 |
6.8 |
6.8 |
0.988 |
1.492 |
|
3 |
4 |
4 |
7.4 |
7.2 |
0.746 |
1.224 |
|
4 |
3 |
3 |
7.6 |
7.6 |
0.814 |
1.002 |
|
5 |
4 |
3 |
7.4 |
7.2 |
0.71 |
1.377 |
|
6 |
4 |
4 |
7 |
6.8 |
0.809 |
0.783 |
|
7 |
4 |
4 |
7.2 |
7.4 |
0.983 |
1.225 |
|
8 |
4 |
4 |
6.8 |
6.8 |
1.097 |
1.269 |
|
9 |
3 |
3 |
7.2 |
7.2 |
1.124 |
1.435 |
|
10 |
4 |
4 |
7.2 |
7.4 |
0.869 |
1.328 |
Table 1 Buffering capacity, pH, Total salivary antioxidant level before and after probiotic administration
Estimation of total antioxidant level
100 microliter of saliva was pipetted into the test tube to which 5% trichloroacetic acid was added. It was then allowed to settle for 5 minutes followed by centrifugation for 10 minutes. 1mL of total antioxidant reagent was added to 100 microliter of the supernatant saliva. This mixture was incubated in hot water bath at 900C for 90 min. Optical density was read at 695nm using spectrophotometer.
Estimation of buffering capacity and pH
Adequate amount of saliva was placed on buffering strips and colour change was noted. pH paper was dipped in the remaining saliva present in vial and change in colour was observed. Interpretation was made based on the colour guide present in the GC Salivary Check kit.
Statistical analysis
Data obtained were statistically analysed by using IBM SPSS version 24. Differences between variables were analysed by “paired t- test”. Descriptive analysis was performed by noting means and standard deviations (Table 2). Variables were plotted on a statistical graph to compare pH, buffering capacity and total antioxidant level before and after probiotic administration.
|
Different parameters |
N |
Mean (SD) |
Mean difference |
95% Confidence interval of the difference |
t |
df |
p-value |
||
|
Lower |
Upper |
||||||||
|
Buffering capacity |
Before |
10 |
3.80 (0.42) |
0.3 |
-0.05 |
0.65 |
1.96 |
9 |
0.08(NS) |
|
After |
10 |
3.50 (0.53) |
|||||||
|
pH |
Before |
10 |
7.16 (0.26) |
0 |
-0.12 |
0.12 |
0 |
9 |
1.00(NS) |
|
After |
10 |
7.16 (0.28) |
|||||||
|
Total antioxidant level |
Before |
10 |
0.94 (0.19) |
-0.3 |
-0.46 |
-0.13 |
-4.08 |
9 |
0.003* |
|
After |
10 |
1.24 (0.21) |
|||||||
Table 2 Statistical analysis
Paired t test
*p<0.05 Statistically Significant, p>0.05 Non Significant, NS
Lactobacillus and Bifidobacterium are two of the most widely used bacteria as probiotics. Use of lactic acid bacteria for therapeutic action in oral cavity was controversial but recent studies have shown its promising result in dental caries, periodontitis, halitosis and several other oral pathologies.12 In the present study administration of probiotics led to no significant change in pH of saliva (Figure 1). This finding corroborate with the findings of Quingru Jiang et al who has also observed no significant change in pH after incorporating probiotic bacteria in biofilm.13 The unaltered pH phenomenon in the present study might be explained by 2 means:
Saliva buffer acts as an important host defence mechanism to control the pH of the mouth environment.14
There was no significant change in buffering capacity of saliva after probiotic intake for 2weeks (Figure 2). This result was in contrast to a study done by Villavicencio in the year 2018 who observed increase in buffering capacity following probiotic administration.15
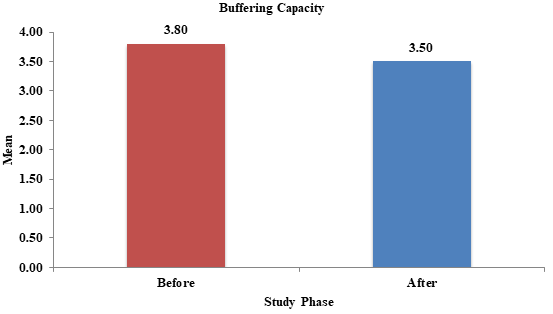
Figure 2 Change in buffering capacity before and after probiotic intake.
p-value: 0.08 (Non-significant).
This result can be due to:
Lastly, the total antioxidant level in saliva increased after administration of probiotics (Figure 3). This finding corroborates with the findings of a study done by Martareli et al which concluded that probiotics leads to increase in plasma antioxidant level.10
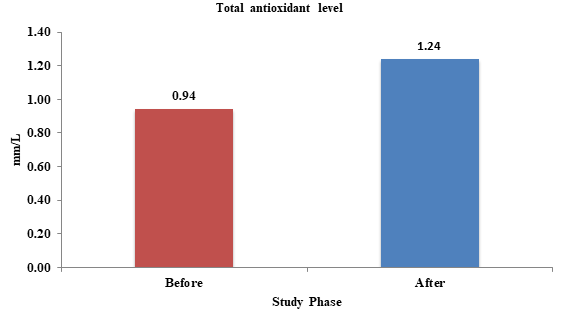
Figure 3 Effect of probiotics on total antioxidant level before and after probiotic administration.
p-value: 0.003 (Significant).
This result can be attributed to:
Till date probiotics were known to balance the microbial ecology. But it acts at cellular level as well. Probiotics increase antioxidant level in the body. These antioxidants prevent free radical generation and thus, interrupt damage to human cell. At the same time it also affects the metabolism of several microbial cells including the ones responsible for oral diseases. Moreover, change in antioxidant level following probiotics can be easily appreciated in saliva. Thus, saliva can be practiced as a routine diagnostic tool for assessing total antioxidant level. It can further be used to evaluate the effect of probiotics on specific antioxidant system related to particular disease.
None.
None.
None.

©2021 Hegde, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.