Journal of
eISSN: 2379-6359


Review Article Volume 17 Issue 1
1Chief of Audiology Nuance Audio TLV Part of Essilor Luxottica, Italy
2Senior Director of Audiology Professional Affairs at Essilor Luxottica and Host of the Hearing Matters Podcast Brenham, Texas, USA
Correspondence: Dr. Douglas L Beck Au. D, Adjunct Clinical Professor of Communication Disorders and Sciences, State University of New York at Buffalo., Tel 210-380-3648
Received: December 02, 2024 | Published: February 3, 2025
Citation: Harel-Arbeli T, Beck DL. An Over-The-Counter (OTC) hearing aid option for people with self-perceived mild-to-moderate hearing loss: Nuance Audio™ Hearing Aid Glasses. J Otolaryngol ENT Res. 2025;17(1):9-14. DOI: 10.15406/joentr.2025.17.00558
Hearing Healthcare is a major issue across the globe. Untreated hearing loss is correlated with anxiety, depression, social isolation, worsening physical health, cognitive decline, decreased communication and more. The most common problem noted among people with self-perceived hearing loss is the inability to understand speech in noise. Nonetheless, the overwhelming percentage of people with hearing loss do not seek help, despite over the counter and prescription-based hearing aid solutions. Access and affordability have been identified as impediments to acquiring hearing solutions. However, in this article we offer often unspoken “real-world” impediments associated with typical hearing aid form factors; stigma and cosmetic concerns. This article introduces the Nuance Audio Glasses solution with a built-in over the counter hearing aid and reviews lab-based results from three clinical pilot studies. Speech in noise results, as well as subjective results from wearers, and the benefits of a cosmetically pleasing and acceptable over the counter hearing aid solution are introduced.
Demographics
The quantity of people with hearing loss in the USA is significantly higher than most people realize. In 2024, the Global Burden of Disease Study1 reports approximately 73 million people in the USA had hearing loss in 2019, representing 22% of the population, or 1 in 5 people. About 90% of them likely have mild to moderate hearing loss. Of the Americans with mild to moderate hearing loss, about 95% are adults aged 18 and above. Humes (2023) used data from the National Health and Nutrition Examination Survey (NHANES) to examine the unmet need for hearing healthcare. Unmet need refers to the percentage of individuals with hearing loss or difficulty hearing who are not current hearing aid users or have never tried hearing aids. According to Humes, and extrapolating from the Global Burden of Disease (GBD) data, 85-90% of those with mild-to-moderate hearing loss fall into this category, resulting in approximately 58-60 million people.
Although reliable historical data is limited, a patent was filed in 1909 for an early device combining hearing aids and spectacles. However, the first manufactured products were not introduced until 1954. By the 1960s, “electronic eyeglass hearing aids” were among the most popular hearing aid form factors, accounting for over half of all hearing aid sales. By the late 1970s and into the 1980s, behind-the-ear (BTE) hearing aids had become the most popular style. On September 7, 1983, it was announced that President Ronald Reagan was wearing custom-made in-the-ear (ITE) hearing aids, which quickly led to ITE hearing aids becoming the predominant fitting style.
In 1996, digital signal processing (DSP) hearing aids became commercially available, featuring smaller, more efficient components and increased processing capabilities. This enabled the development of ear-level hearing aids with advanced technology in increasingly compact custom-made designs, and the combined eyeglass-hearing aid products largely faded from the market.
Everett2 reported in “Over the Counter (OTC) Hearing Aids in the USA” for the National Council on Aging (NCOA) that OTC hearing aids are intended for adults with self-perceived mild to moderate hearing loss and can be purchased without a hearing exam or prescription. The FDA’s final rule on OTC hearing aids, effective October 17, 2022, aimed to improve accessibility and affordability, while fostering innovation and competition in the hearing aid market. As a result, hearing aids are now widely available online, in pharmacies, electronics stores, and other retail outlets. Nonetheless, Beck and Harvey3 noted that higher-quality OTC hearing aids still cost between $1,000 and $2,950 per pair.
Over the counter hearing aids were expected to increase hearing aid adoption, targeting the 58-60 million Americans with an unmet need for hearing healthcare. However, the impact appears to be less transformative than anticipated. Although access to hearing aids has improved following the FDA rule on OTC hearing aids, the prescription-based market has not been significantly disrupted.
Beck and Harvey3 highlighted the probable “third leg of the stool”—cosmetics and stigma—as a major barrier. They observe that many people do not want to be associated with traditional hearing aids. These devices are often perceived as indicating old age or are simply considered unattractive, which aligns with the broader societal values on physical appearance, youth, beauty and fashion.
Cosmetics and stigma
Wallhagen4 documented associations between hearing aids, ageism, and perceptions of disability. Alcido (2024) reported that “social stigma” remains a major deterrent, with 63% of Millennials, 47% of Gen Xers, and 41% of Boomers agreeing that stigma exists around hearing aids. Novak (2024) echoed this sentiment, finding that resistance to hearing aids is largely rooted in associations with old age. While the introduction of OTC hearing aids was intended to improve access and affordability, most OTC and prescription hearing aids still share similar form factors, and neither category has significantly addressed cosmetic concerns or the associated stigma.
Another barrier is physical fit and comfort. Oosthuizen et al.,5 noted that discomfort, irritation, or pain from poorly fitting hearing aids or ear molds can lead to reduced use or non-use. Franks and Timmer6 also cited lack of comfort as a reason for abandonment of hearing aids.
Finally, issues with perceived benefit are prevalent. McCormack and Fortnum7 found that participants frequently expressed dissatisfaction with the "value" of hearing aids, particularly in noisy environments. This finding is consistent with more recent insights from Franks and Timmer6 who reported that many users perceive limited benefits from hearing aids.
An alternative OTC form factor
Nuance Audio™ Hearing Aid software uses the compatible wearable electronic smart glasses, Nuance Audio™ Hearing Glasses™, to offer an innovative, updated version of eyeglass-integrated hearing assistance available in high-fashion frames, with an OTC hearing aid software feature. The smart glasses frames has six microphones and a sound processor. The SaMD hearing aid feature provides amplification like the overall concept from Urbanski et al, .8 Their study addressed NAL-NL2 objectives and was aimed at fitting most older Americans with mild to moderate sensorineural hearing loss due to presbycusis. Urbanski et al. report their fitting paradigm could provide efficacy comparable to audiology best practice protocols, making it suitable for OTC preset-amplification profiles to meet the amplification needs of most individuals with mild to moderate hearing loss. Nuance Audio™ Glasses offer the same open-ear output gain for each ear simultaneously and uses a unique multiple microphone array to allow spectral shaping.
The Nuance Audio™ Hearing Aid incorporates noise reduction and beamforming algorithms, with user control options. Options include a smartphone app, a physical volume and on/off button on the temples, and an optional remote control. As envisioned decades ago, the beamforming algorithms use spatial separation between microphones and advanced software processing to achieve a high directivity index, maximizing the signal-to-noise ratio for sounds originating in front of the wearer Figure 1. The Nuance Audio™ Glasses transmit sound to each ear through tiny speakers embedded in the frames, without requiring anything to be worn in the ear, or to enter the ear canal, making them the first true open-ear air-conduction OTC hearing aid.
The benefits afforded the wearer via the Nuance Audio™ Hearing Aid and Glasses include:
Report 1
Harel-Arbeli (2024a)9 reported a pilot study conducted by an external research laboratory titled “Speech-in-Noise (SIN) Evaluation Comparing Nuance Audio™ Hearing Aid to Two Premium Prescription Hearing Aids.” The SIN assessment tool was the Hebrew version of the Matrix test (Oldenburg sentences)1. Participants included nineteen adults (8 females, 11 males), with an average age of 69 years (ranging from 41 to 88 years) all with mild-to-moderate sensorineural hearing loss and all experienced hearing aid users. The SIN test protocol involved three speakers. The target speech was delivered via Speaker One, located one meter in front of the subject. Speech Spectrum Noise (SSN) was delivered through Speakers Two and Three, positioned one meter from the subject at 90 and 270 degrees (i.e., perpendicular).
Two popular, commercially available premium prescription hearing aids (i.e., Product A and Product B) were fitted bilaterally based on the first fit protocol and tested with directional settings on. The Nuance Audio™ Hearing Aid was set by an audiologist to the optimal preset for the subject's hearing loss, programmed to conversation mode with directional settings on. SIN testing was administered to determine the SNR-50 (the signal-to-noise ratio at which the user can recognize and repeat 50% of the words2). Each SNR-50 was determined using twenty 5-word sentences presented at various SNR levels to establish the SNR-50 for each of the randomly ordered conditions (condition one - unaided, condition two - Nuance Audio™ Hearing Aid, condition three - Product A, condition four - Product B).
Harel-Arbeli reported the aided SNR-50s were generally better than the unaided SNR-50s, with the best aided SNR-50s obtained using Nuance Audio™ Hearing Aid. A repeated measures ANOVA was conducted to determine whether statistically significant differences in performance across the four conditions were present. Mauchly’s Test of Sphericity indicated that the assumption of sphericity was not violated, thus, no correction was applied. The repeated measures ANOVA demonstrated a significant main effect of listening condition (Nuance Audio™ Hearing Aid, Product A, Product B) on the outcome variable (SNR-50), with 65% of the variance explained by the listening condition. Post-hoc pairwise comparisons indicated that each of the three aided conditions was significantly different from the unaided condition. Products A and B yielded comparable outcomes (with no significant differences between them) while Nuance Audio™ Hearing Aid performed significantly better than either regarding SNR-50 measures. See Figure 2 for results.
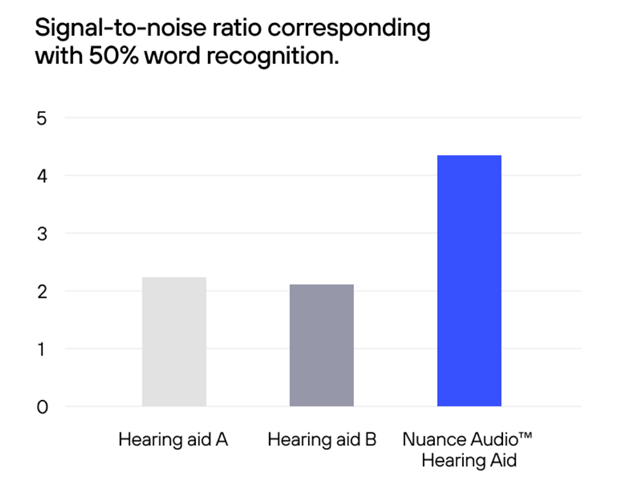
Figure 2 Improvement in SNR-50 for Hearing aids A, B, and Nuance Audio™ Hearing Aid.
Nuance Audio™ Hearing Aid significantly improved speech-in-noise ability. Nuance Audio™ Hearing Aid out-performed the two commercially available prescription hearing aids.
Report 2
Harel-Arbeli(2024b)10 reports 43 participants of average age 67 years (range 30 to 87 years, 17 females, 26 males) using Nuance Audio™ Hearing Aid in real-world scenarios. The average four-frequency pure tone average (4FPTA) was approximately 38dB. Thirteen participants had previous hearing aid experience. This study was approved by the Ethics Committee of Reichman University and all participants signed an informed consent form.
At screening, each participant answered “yes” to at least one of the following statements from the FDA11 signs of mild to moderate hearing loss:
Participants were fitted with Nuance Audio™ Hearing Aid using the on-board calibrations (own voice and feedback) and the most comfortable preset (gain/frequency settings). The Hebrew Matrix speech-in-noise test was conducted similarly to the protocol described in Report 1. Additionally, subjective “real world” ratings were acquired in simulated real-life acoustic situations, including conversational speech with the audiologist in quiet and simulated noise with two-talker babble from one speaker and music from the other. After experiencing the simulated real-life acoustic situations subjects were asked to rate Nuance Audio™ Hearing Aid based on their own listening experiences in quiet and noise in accordance with the statements below, using a Likert scale (1 = completely disagree, 3 = neutral, 5 = completely agree).
STATEMENT ONE: I can hear the conversation “clearly”3 with the glasses.
STATEMENT TWO: Sound quality is good, without adverse effects.
Subjects were also asked to rate changes in listening effort, also on a Likert scale (1 = worse, 3 = neutral, 5 = much better). The specific statement they responded to was:
“Did you feel a change in listening effort while using the glasses?”
Results of the speech-in-noise test demonstrated the average unaided (glasses turned off) vs. aided (glasses turned on) SNR-50 improvement was 3.49 dB. A paired t-test determined the significance of the improvement. The t-statistic of 15.89 with a p-value of less than 0.001 demonstrates a highly significant difference between the Nuance Audio™ Hearing Aid turned off and on.
Regarding the subjective “real world” ratings per the Likert scales described above, the results are:
Table 1 Therefore, the Nuance Audio™ Hearing Aid demonstrated significant improvements in SNR-50 measurements, indicating significant improvements in the ability to understand speech-in-noise. The Nuance Audio™ Hearing Aid was determined to be significantly better than neutral across all three subjective “real life” situations. Overall, the results from Harel-Arbeli (2024b)10 indicate that Nuance Audio™ Hearing Aid were positively perceived across all evaluated aspects, confirming their effectiveness and user satisfaction.
|
Measurement |
Mean |
Median |
Standard deviation |
t Statistic |
P Value |
|
Speech clarity |
4.79 |
5 |
0.38 |
30.77 |
<.001 |
|
Overall sound quality |
4.84 |
5 |
0.41 |
29.14 |
<.001 |
|
Release from listening effort |
4.13 |
4 |
0.9 |
14.51 |
<.001 |
Table 1 “Real world” rating and statistic data regarding the subjective experience with Nuance Audio™ Hearing Aid
Report 3
The Cox and Alexander12 7-item questionnaire, titled “International Outcome Inventory for Hearing Aids (IOI-HA),” was used to evaluate and differentiate hearing aid treatments. The original IOI-HA is “…general enough to be appended to other outcome measures that might be planned in a particular application and will provide directly comparable data across otherwise incompatible projects.”
Briefly, the IOI-HA items are referred to as:
Item 1 – Use
Item 2 – Benefit
Item 3 – Residual Activity Limitation
Item 4 – Satisfaction
Item 5 – Residual Participation Restriction
Item 6 – Impact on Others
Item 7 – Quality of Life
As such, the IOI-HA is an ideal tool to compare Nuance Audio™ Hearing Aid and Glasses to traditional form-factor hearing aids.
Twenty-three adult subjects (43% female, 57% male, ages 55 to 82 years) with self-perceived mild-to-moderate sensorineural hearing loss completed the IOI-HA after using Nuance Audio™ Hearing Aid for 14 days. All subjects were habitual users of their own glasses and maintained an active social life. They were instructed to replace their own prescription glasses with Nuance Audio™ Hearing Aid (with appropriate prescription lenses) and to use them daily.
The Table 1below presents normative data from the IOI-HA and Nuance Audio™ Hearing Aid along with results from an independent two-sample t-test. Results show no significant difference in performance between prescription hearing aids and Nuance Audio™ Hearing Aid in the benefit and quality of life items (items 2 and 7). However, all other items (IOI items 1, 3, 4, 5, 6) indicate Nuance Audio™ Hearing Aid scored significantly better than the published norms for traditional hearing aids Table 2.
|
Item |
Traditional HA (n=71) |
Nuance audio (n=23) |
t |
p |
|
Use |
3.73 (1.17) |
4.22 (0.85) |
2.18 |
0.03 |
|
Benefit |
3.39 (0.98) |
3.74 (1.29) |
1.19 |
0.25 |
|
Residual activity limitation |
3.40 (0.95) |
4.30 (0.82) |
4.39 |
0.001 |
|
Satisfaction |
3.20 (1.21) |
3.91 (1.44) |
2.13 |
0.04 |
|
Residual participation restriction |
3.57 (1.13) |
4.57 (0.73) |
4.93 |
0.001 |
|
Impact on others |
3.79 (1.13) |
4.70 (0.63) |
4.85 |
0.001 |
|
Quality of life |
3.19 (0.93) |
3.61 (1.23) |
1.50 |
0.15 |
Table 2 IOI-HA questionnaire results: Mean and standard deviation for prescription hearing aids (Cox, 2003) and Nuance Audio™ Glasses, and statistic t-test results
The graphs below present each of the IOI-HA questions and the data obtained in the study.
1“International matrix tests have achieved wide recognition as speech testing procedures in noise for improved diagnostics and in the use of studies especially in the field of hearing systems. The international matrix tests are now available in 19 different languages, covering over 60% of the world's population. An automated process allows for accurate results - across all language barriers. International studies also require the use of procedures whose results are comparable with each other. For this reason, special care was taken in the development of the international matrix tests to ensure that the test procedures in the various languages are highly comparable.” https://www.hz-ol.de/en/matrix.html)
2Beck & Benitez (2019) described the SNR-50 as a rapid protocol which accurately determines the SNR required to obtain a speech in noise score of 50 percent correct, referred to as the “SNR-50.” They report establishing the SNR-50 is a similar protocol to obtaining a pure-tone threshold. The primary speech signal is held constant (at the most comfortable loudness, MCL) while (noise) ascends and descends. The SNR-50 is identified when the subject repeats half of the target words (50 percent) correctly.
3Note: Although “hearing clearly” is subjective, that term was used and subjects responded without further discussion as to the definition of “clearly.”
In the United States, there is a significant unmet need for hearing healthcare. Although estimates vary by source, it is generally reported that 1 in 5 people in the U.S. has hearing issues, and only 10-15% of individuals with hearing difficulty use hearing aids.
Despite the FDA’s 2022 Over-the-Counter (OTC) provisions aimed at improving access and affordability, unresolved issues persist, especially regarding stigma, the appeal of current hearing aid form factors and designs, and affordability.
Many of the most common prescription and OTC hearing aids use familiar form factors (e.g., BTEs, RITEs, RICs) which are often associated with undesirable characteristics, making these devices less attractive to wear.13 Additionally, if the physical fit of the hearing aid or ear mold causes discomfort, irritation, or pain, this can further discourage consistent use, potentially leading to reduced use or even non-use.
EssilorLuxottica introduced an updated alternative to traditional prescription and OTC hearing aids with the Nuance Audio™ Hearing Aid software feature. They also developed a sophisticated SaMD using four presets, noise reduction, and advanced beamforming, executed within attractive, fashion-forward smart eyeglass frames, referred to as Nuance Audio ™ Glasses. The Nuance Audio Glasses have multiple microphones, open ear speakers, and a rechargeable battery.
We recommend a comprehensive hearing health assessment by a licensed hearing care provider for individuals concerned about their own or a loved one’s hearing health. Hearing health is crucial for hearing, listening, communication, hearing in noise, tinnitus management, balance, cognitive function, mental health, personal and social relationships, social engagement, independence, financial stability, quality of life, and more. However, for those who have perceived mild to moderate hearing loss who seek an OTC hearing aid that provides benefit for speech in noise and is physically comfortable and cosmetically pleasing, the Nuance Audio™ Hearing Aid represents an innovative and exciting option. This article reviewed three reports showing that Nuance Audio™ Hearing Aid using Nuance Audio™ Glasses improves Speech-in-Noise ability, delivers beneficial results regarding speech clarity in challenging listening situations, and reduces listening effort. The Nuance Audio™ Hearing Aid performed significantly better than published norms for prescription hearing aids across five of the seven International Outcomes Inventory items, matching prescription hearing aids in the remaining two. Notably, Nuance Audio™ Glasses are not associated with the negative cosmetic issues and stigmas commonly linked with traditional OTC and prescription hearing aids form factors.
None.
Dr. Douglas L. Beck Au.D. is a paid consultant for Essilor Luxottica.

©2025 Harel-Arbeli, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.