Journal of
eISSN: 2379-6359


Research Article Volume 10 Issue 4
ENT Department of Hospital Italiano de Buenos Aires, Argentina
Correspondence: Elian Garcia Pita ENT Department of Hospital Italiano de Buenos Aires, Palestina 561, Buenos Aires, Argentina, Tel +54 9 11 60529874
Received: May 29, 2018 | Published: August 17, 2018
Citation: Ruggeri C, Lopez S, Molachino R, et al. Neural tumors of the head and neck. J Otolaryngol ENT Res. 2018;10(4):252-258. DOI: 10.15406/joentr.2018.10.00356
Objective: 1. Describe the clinical characteristics, diagnostic methods, and surgical technique indicated in neural tumors located in the head and neck. 2. Determine the rate of preservation of nerve function and recurrence after surgical treatment.
Design: Descriptive and retrospective
Materials and methods: We included all patients with a histological diagnosis of neural neoplasms located at the head and neck that were evaluated and treated at the ENT department of the Hospital Italiano de Buenos Aires, between March 1998 and December 2017. We excluded patients with neural tumors located at the endocranium without extracranial involvement and those with possible clinical / imaging diagnosis of a nerve tumor, in whom control was indicated or who did not want to undergo surgery. 3 types of surgical techniques were performed. The total resection consisted in the radical excision of the neural neoplasm, almost always with the sacrifice of the nerve. The subcapsular resection, or intracapsular enucleation, consisted in determining with the nerve stimulator the area of lesser function in the tumor (less nerve fibers) and then, making an incision in order to resect the tumor subcapsularly, trying to preserve as much as possible nerve fibers. In the partial resection, the exeresis was subtotal, leaving tumor in addition to the capsule. It was considered that the preservation of the function after the surgery was total when the nerve that originated the neoplasm had no deficit.
Results: Twelve patients had histological confirmation of neural tumor. Ten were neurilemoma and two solitary neurofibroma. Four originated at the facial nerve in the parotid, three of them were treated with intracapsular enucleation technique and one with complete resection. Six tumors were located at the neck. Three of them, in the retro-tyloid carotid space, one in the submaxillary region and two in the supraclavicular fossa: all were completely resected. One tumor was located at the nasal cavity and another tumor was originated from the endocranium, and extension to nasal cavity: both from the V cranial nerve. The resection was total in one and partial in another.
Conclusion: It is important to make a preoperative diagnosis of neural neoplasia, and to determine the nerve that is compromised. The location at the neck and especially the imaging characteristics of these lesions can be used to make the preoperative diagnosis. Anticipating the neurological deficit that can be caused by the surgery, in case of symptomatic patients it can be decided to indicate a radical technique or intracapsular enucleation in order to preserve as much as possible of nerve function and improve the quality of life. The total preservation of nerve function was 58.33% (7/12) and there were no recurrences in the 11 patients treated with radical resections and intracapsular enucleation.
Keywords: schwannoma, extracranial, head and neck
Schwannomas or neurilemomas are nerve tumors that have a capsule and they originate between the 25 to 45% of the cases in nerves of head and neck.
Neurofibromas are rarer, they don´t have a capsule and can present as a solitary or multiple tumor, being part of the neurofibromatosis syndrome. Both kinds of tumors are predominantly benign.
The treatment in case of symptomatic tumors is surgical, and there are two prevalent surgical techniques. The radical resection of the tumor with preservation or sacrifice of the nerve and the intracapsular enucleation, which tries to preserve nerve fibers to preserve function as much as possible.
A descriptive and retrospective study.
We included all patients with a histological diagnosis of neural neoplasms located at the head and neck, than were evaluated and treated at the ENT department of the Hospital Italiano de Buenos Aires, between March 1998 and December 2017.
We excluded patients with neural tumors located at the endocranium without extracranial involvement and those with possible clinical/imaging diagnosis of a nerve tumor, in whom control was indicated or who did not want to undergo surgery. The age and sex of the patient were recorded, symptoms, location of the tumor, possible nerve of origin, type of surgical approach, characteristics of the resection, results and complications.
It was considered that the function preservation after surgery was total when the nerve had no deficit. To evaluate the postoperative function of the facial nerve, the House-Brackmann scale was used (Table 1). The function mass evaluated up to 6 months.
Grade |
Definition |
I |
Normal symmerricalfimction in all areas |
II |
Slighr weakness noticeable only on close inspection Complete eye closure with minimal efforr Slight asymmetry of smile with maximal effort Synkinesis barely noticeable, conrracture, or spasm absent |
III |
Obvious weakness, but nor disfiguring May not be able to lift :11ebrow Complete eye closure and strong but asymmetrical momh movemenr with maximal effort Obvious, but not disfiguring synki11esis, mass movement or spasm |
IV |
Obvious disfiguring wealmess Inability ro Ii.ft brow Incomplete )'e closure and asymme11y of mourh with maximal effort Severe synkinesis, mass movement, spasm |
V |
Motion barelyperceptible Incomplete ye closure, slighr movement corner mouth Synkinesis, cont1·act1ll'e, and spasm usually absent |
VI |
No movement, loss of lone, no synlrinesis, conrracrure, or spasm |
Table 1 House Brackmann
Three types of surgical techniques were performed. The total resection consisted in the radical excision of the neural neoplasm, almost always with the sacrifice of the nerve. The subcapsular resection, or intracapsular enucleation, consisted in determining with the nerve stimulator the area of lesser function in the tumor (less nerve fibers) and then, making an incision in order to resect the tumor subcapsularly, trying to preserve as much as possible nerve fibers. In the partial resection, the exeresis was subtotal, leaving tumor in addition to the capsule. In all cases, intraoperative biopsies were done in order to confirm that were neural and benign tumors.
Fifteen patients with probable neural tumors located at the head and neck region were evaluated. Three of them, were excluded because they did not have histological confirmation: control (no surgery) was indicated in 2 (one patient was 81years old with bad general condition, who had a vocal cord paralysis and another was a 68 year sold patient with a small asymptomatic tumor located in the retro-styloid carotid space). Another patient, was 76 years old patient, and had a voluminous retro-styloid tumor; he refused surgery.
Twelve patients had histological confirmation of neural tumor. Five of them, were women and 7 were men. The youngest patient was 16 years old and the oldest was 71. Ten patients had histological diagnosis of neurilemoma and two of solitary neurofibroma.
Four women presented nervous tumors at the parotid gland (3 neurilemomas and 1 neurofibroma). Two were located at the superficial lobe and two at the superficial and deep lobe of the gland. All patients consulted because of the presence of a parotid tumor, without alteration of facial nerve function. In two cases, a presumptive preoperative diagnosis of neoplasia of facial nerve was made by computed tomography and magnetic resonance with contrast. Two patients were evaluated only by ultrasound, obtaining non-specific information of the presence of a solid tumor, and the diagnosis was intraoperative, because in one of them was impossible to differentiate the trunk of the facial nerve from the neoplasm, and in the another one when it was found that the tumor was originated from the buccal and zygomatic branches of the facial nerve. All four patients were studied by fine needle aspiration with ultrasound guidance; neurilemoma was confirmed only in one of them.
In three patients, a subcapsular resection of the tumor was performed in order to preserve the facial nerve function and in another the resection was complete (neurofibroma originated in a secondary anastomotic branch).
In three patients, a facial nerve monitor was used, and the incision was made in order to make the subcapsular dissection in an area that did not generate stimulation; in one, a mastoidectomy was performed to achieve a better expose of main facial nerve trunk, combined with the parotidotomy.
The function of the facial nerve was completely preserved in 2 patients (Brackmann I), another one had a slight dysfunction in the mandibular branch (Brackmann II: minimal asymmetry of movement in the mouth) and in another, nerve function was more affected (Brackmann III). The average period of control in this group was 4 years, and no recurrence was detected in the MRI (Figure 1–3).
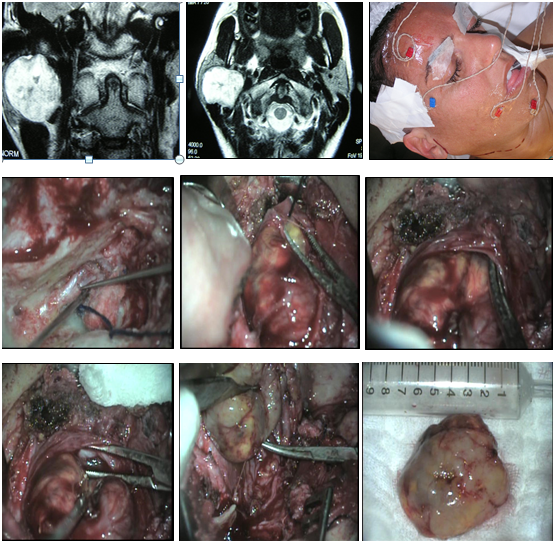
Figure 1 Neurilemoma of the facial nerve at the parotid gland. Mastoidectomy, parotidotomy and intracapsular tumor resection
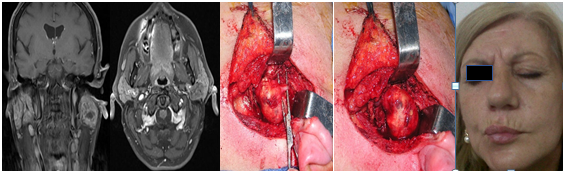
Figure 2 Schwannoma of the facial nerve at the parotid gland. Intracapsular resection with preservation of nerve function.
Six patients had neurilemomas located at the neck. The symptom was cervical tumor in all cases, and also disphagia in two of them. In three cases, the tumor was located at the retro-styloid carotid space. They were evaluated with tomography and magnetic resonance with contrast, and in two of them a fine needle aspiration was done with ultrasound guidance; the result was non-specific. There were not alterations in mobility of vocal cords.
In two bulky neoplasms, a displacement of the amygdala and soft palate towards the midline was observed. A transcervical approach was performed in all patients, and in two of them the exposure was extended by dissecting the posterior sector of the submaxillary gland, which was preserved.
Two tumors were originated from the cervical sympathetic chain and in another case the nerve of origin could not be specified. The resections were total and two patients had a postoperative Claude-Bernard-Horner syndrome, and one also had a “first bite” syndrome (pain in the parotid gland or in the temporomandibular joint when chewing the first time in each ingestion that decreases in successive bites) (Figure 4–6).
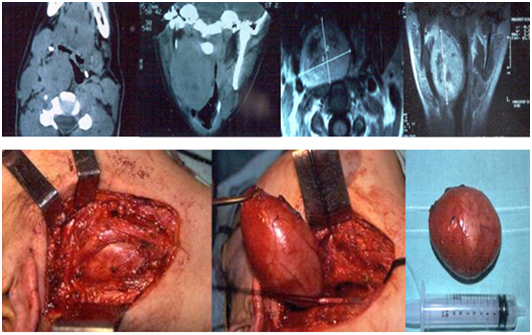
Figure 4 Neurilemoma originated at the cervical sympathetic chain, in the retro-styloid carotid space. Transcervical approach: complete resection.

Figure 5 Schwannoma originated at the cervical sympathetic chain in the retro-styloid carotid space. Transcervical approach: complete resection.
A neurilemoma was located at the submaxillary region and it was originated from the glossopharyngeal nerve. It had been studied by ultrasound and contrasted tomography. The pre operatory cytological study was nonspecific. It was completely resected preserving the submaxillary gland, without any post operatory neurological deficit.
Two tumors were located at the supraclavicular fossa. The way of presentation of them was a tumor at the region of the neck. They were studied by tomography and MRI. The fine needle aspiratorion was non-especific. They were completely resected by a cervical approach. The possible nerves of origin were branches of the cervical sympathetic chain. There was no post operatory neurological deficit. There were no recurrences during the follow-up (5 years) (Figure 7).
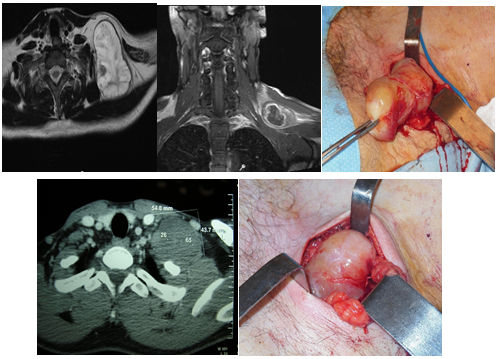
Figure 7 Solitary neurofibroma (above), and neurilemoma (below), originated at branches of the cervical sympathetic chain. Complete resection.
Two patients had neurilemomas located at the nasal cavity. One of them, consulted for left nasal obstruction and another for epistaxis and bilateral disturbance of visual acuity.
They were evaluated by tomography and MRI. A neoplasm was located at the anterior area of the left nostril, and another had an endocranial origin, and had extension to the nasal cavity, eroding the lamina cribosa. In both cases, a biopsy was performed, and the diagnosis was schwannoma. The nerve of origin in both cases was the trigeminal. A neoplasm was completely resected by a sublabial approach, and in the other case, through a craneonasal approach. When performing the anterior craniotomy, it was found that the tumor was englobing the optical chiasm and the proximal area of both optic nerves, so a partial and conservative resection was performed. The patient with the nasal cavity neurilemoma had no neurological deficit.
The post operatory controls did not detect a recurrence during a 5-year follow-up in the case of the nasal tumor, and the patient with the intracranial neurilemoma with nasal extension had a better quality of life and improvement in visual acuity during the 2-year follow-up with residual tumor (Figure 8).
The total preservation of neurological function was 58.33% (7/12). There were no recurrences in the 11 patients treated with radical resections and intracapsular enucleation.
Neural tumors are infrequent, accounting for 1% of neoplasms of the head and neck. The Schwann cells that form the nerve sheath are the origin of neurilemomas and neurofibromas. They can originate at the sheath of any nerve, except the optic and olfactory nerve (they don´t have Schwann cells). At the head and neck area, these tumors can grow from the VIII and X cranial nerves, cervical sympathetic chain, and less frequently VII, IX, XI, XII cranial nerves, brachial plexus, and maxillary or ophthalmic branches of trigeminal nerve at the paranasal sinuses. At the neck, the most common place of occurrence is the parapharyngeal space, and the most frequent nerves of origin are the X cranial nerve and the sympathetic cervical chain.
Zheng et al described, in a retrospective study, 91 neurilemomas of the parapharyngeal space. The 45.7% (42 cases) were neoplasms originated at the cervical sympathetic chain, and the rest were originated at the X cranial nerve.1
These tumors can produce displacement of the pharynx, tonsils, soft palate, presenting as a lateral tumor of the neck or a pseudoparotidomegaly. In extensive neoplasms, it is difficult to determine the nerve of origin. They have slow growing and an expansive pattern, displacing the axons. This would allow separating the tumor from the nerve and preserving its function, although many times, especially in extensive cases, this is difficult to perform. It would also explain the high frequency of large neurilemomas, without nerve dysfunction. In our series, three neurilemomas were located at the retro-styloid carotid space.
The incidence of tumors of the facial nerve in the parotid is 0.2 to 1.5%. Eneroth et al.,2 between 802 parotid tumors, found two neurogenic neoplasms, and in a review of 700 parotidectomies, Nussbaum3 found a case of neurilemoma of the facial nerve.
In our study, we had 4 neural tumors originated at the VII cranial nerve, among 70 patients operated for parotid tumors (5.71%). The diagnosis of intraparotid facial neurilemoma is difficult if there are no signs of paralysis, what is uncommon at the extra-temporal location due to the possibility of growing in an extensible space.
Intratemporal schwannomas, cause early facial paralysis, due to the compression of the nerve at the bone nerviduct. Neurilemomas originated at the trigeminal nerve are very infrequent. They can originate from the Gasser ganglion or from any of the three divisions of the fifth cranial nerve; therefore, they can occupy the posterior and / or middle floors of the skull base and even extracranial compartments. Those woth intracranial origins represent 0.07 to 0.3% of intracranial tumors and 0.8 to 5% of intracranial Schwannomas.4,5
The presumptive preoperative diagnosis of neurilemomas can be made taking into account the location and imaging characteristics of these tumors. The heterogeneous aspect, solid and cystic, with little affinity for contrast is common to observe. By MRI, they are isointense in T1 and hyperintense heterogeneously in T2. In some cases the neoplasm can be seen originating from a nervous trunk.
The cytological study of the material obtained by fine needle aspiration is rarely satisfactory for the diagnosis (20-30.3%)1–6 due to the coexistence of zones of cystic degeneration, hemorrhagic necrosis, and calcifications. Only in one patient of our series the preoperative cytological diagnosis of neurilemoma was made.
Some studies report that the separation of the internal carotid artery (ICA) from the internal jugular vein (VYI) at the parapharyngeal space is frecuently seen in neoplasms originated at the X cranial nerve and would not occur in schwannomas of the cervical sympathetic chain, which would displace both vessels anterolaterally. However, Sato and colleagues reported that 5 of 12 cervical sympathetic neurilemomas produced separation between the ICA and the VYI, which would further complicate the diagnosis of the nerve of origin.7 The diagnosis of Schwannoma is confirmed many times by intraoperative biopsy and deferred anatomopathological study.
The treatment is surgical. Morbidity and the possibility of an incomplete resection increase with the increase in size of the neoplasm, so it is convenient to operate early in symptomatic and young patients. Although it is difficult, it is important to try to identify preoperatively the nerve in which the neoplasm originates. Because frecuently the tumor cannot be separated from the nerve, the possible dysfunction that surgical resection can produce must be considered. Neurilemmomas grow slowly, approximately 1mm per year. Except for rapidly growing lesions, which suggest malignant transformation, in extensive tumors in which resection may cause significant morbidity, or in elderly patients, periodic clinical monitoring may be indicated.
The incidence of malignant schwannomas and the rate of malignant transformation of benign neurilemomas is not reported in the literature. The review of malignant intracranial tumors originated at the neural sheath suggests that many of them occur sporadically, rather than from pre-existing neurilemomas. The majority of malignant neural tumors are associated with neurofibromatosis type I. Based on these evidences, the rate of malignant transformation of extracranial solitary schwannomas is considered to be very low. The surgery should be indicated taking into account the risk and benefit of the operation, the preoperative symptoms and the anticipated neurological deficit that the intervention would produce.8
The intracapsular resection of these neoplasms can be considered when the injury of the originating nervous trunk can cause an important morbidity, and to decrease the severity and the incidence of complications not related to the sacrifice of the nerve involved. Sato reported an incidence of postoperative complications not related to the compromise of the involved nerve of 0% after subcapsular resection versus 40% after total tumor resection. In the same study, the incidence of the “first bite” syndrome (hyperactivation of the parasympathetic system that would cause excessive contraction of the myoepithelial cells of the parotid gland) produced in resections of cervical sympathetic chain neurilemomas was lower with intracapsular enucleation technique (40%) that in total resections (100%), the authors attribute it to the preservation of sympathetic chain fibers that go from the superior cervical ganglion to the parotid gland.
In the same study they reported that they could only perform enucleation in 7 of 21 neurilemomas of the parapharyngeal space, and complete preservation of the function could not be possible in any of them. Two patients with neurilemomas of the X cranial nerve had unilateral vocal cord paralysis in the postoperative period, but none alteration of the tone was sen, presenting slight cordal atrophy and mild dysphonia. This was attributed to the preservation of the continuity of the nerve fibers with the enucleation technique.7
Zheng et at.,1 treated 91 patients with extracranial neurilemomas using the intracapsular enucleation technique. He reported 26.2% vocal cord paralysis, and 33.3% Horner syndrom.1
These numbers were much lower than those mentioned by Cavallaro and colleagues in a meta-analysis (87.5% of postoperative dysphonia in schwannomas of the pneumogastric nerve and 80% of Horner's syndrome in tumors of the cervical sympathetic chain).9 Netterville treated 16 vagal tumors with intracapsular enucleation, 15 of them had normal or close to normal voice one year after surgery (8 normal function, 3 partial mobility and normal voice, and 4 had chordal immobility with normal or close to normal voice). Only one patient required a vocal cord medialization with arytenoid adduction. He also treated with enucleation three neurilemomas of the cervical sympathetic chain, all three had postoperative Horner syndrome but they improved significantly at 6 months post operatory. Four brachial plexus tumors were enucleated without suffering anyalterations in arm function.10
In another study, they compared two groups of patients with neurilemomas of the X cranial nerve treated with enucleation9 and with radical resection.13 22% of patients had no paralysis in the first group vs 100% paralysis in the second. Recurrence was 33% (3/9) in patients treated with enucleation and 0% (0/23) in whom a radical resection was made.11
The incidence of postoperative recurrence with the enucleation technique is poorly understood. Ljichi12 reported 0% recurrence in 15 neurilemomas treated with enucleation, with a follow-up of 2.7 years; two other studies did not detect recurrences during follow-up in patients operated with intracapsular enucleation.13,14
In our study, the rate of total preservation of neurological function (7/12) was partly due to the intra-surgical finding that the tumor was originated in secondary nerve branches (4/7).
The preoperative function of the facial nerve is an aspect to consider at the time of selecting the surgical technique in schwannomas of the VII cranial nerve. If the degree of facial dysfunction is important, total resection of the tumor and nerve reconstruction at the same surgical time using an auricular graft or other nerve through a terminal-terminal anastomosis is the best option. If this is not possible due to the extension of the neural compromise, a facial hypoglossal anastomosis can be done. If the function of the facial nerve is normal or the dysfunction is minimal, the type of resection can be decided at the time of surgery. If the tumor is attached to the nerve and it can be dissected, it is better to try a complete resection with preservation of the facial nerve function, and if it can´t be dissected; only a biopsy or a subcapsular enucleation can be performed.15–19
Neurofibromas do not have a capsule and cause a fusiform dilation, incorporating the axons inside the tumor, making impossible to preserve the nerve. They can present as solitary tumors or associated to one of the two forms of neurofibromatosis.
We consider that is very important to make a presumptive preoperative diagnosis of neural disease, and although it is difficult, determine the nerve that causes the tumor. The location at the neck and especially the imaging characteristics of these tumors can be used to make the preoperative diagnosis.
Anticipating the neurological deficit that could be caused by surgery, in symptomatic patients it can be decided to indicate a radical technique or intracapsular enucleation in order to preserve maximum function and improve the quality of life of the patient, reducing complications.
In our study, the total preservation of neurological function was 58.33% (7/12) and there were no recurrences in the 11 patients treated with radical resections and intracapsular enucleation.
None.
The author declares there are no conflicts of interest.

©2018 Ruggeri, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.