Journal of
eISSN: 2379-6359


auditory brainstem response, micrornas, sgs
ABR, auditory brainstem response; SNHL, sensorineural hearing loss; PTA, pure tone audiometry; SV, stria vascularis; ROS, redox oxidative species; MELAS, myopathy, encephalopathy, lactic acidosis and stroke-like episodes
We would like to bring to the readers’ attention a specific aspect of Auditory Brainstem Response (ABR) in patients with Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis and Stroke-Like Episodes (MELAS). In the literature, it is often reported that the outcome of ABR testing is normal in these patients, even when they suffer from severe to profound SensoriNeural Hearing Loss (SNHL); however, some authors observed absence of ABR waves in mild forms of SNHL.1‒6 How can this disagreement in observations be explained?
We observed a MELAS patient who came to our Clinical Center for moderate SNHL. The ABR from this patient displayed waves (Figure 1) with amplitude and latency too reduced to be considered within the normal range, but not completely absent as it would be expected considering his auditory threshold in pure tone audiometry (PTA).
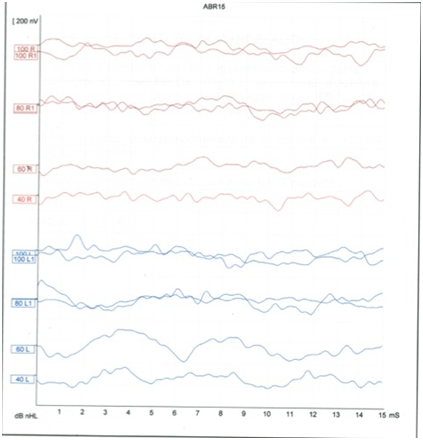
Figure 1The image shows the ABR with no structured waves in both sides. The signal is not transmitted at 40 and 60 Db, at 100 dB in the left side seems to appear a wave I.
In an attempt to explain this observation, we reviewed the literature on the temporal bone aspect of MELAS patients. Loss of Spiral Ganglions (SGs) and atrophy of Stria Vascularis (SV) are typical findings in temporal bone of patients with MELAS even when the Organ of Corti is entirely preserved.
In SGs and SV mitochondria are abundant,7 as they are necessary to support the high metabolic functions of these structures. In MELAS, mitochondria that have been damaged (by deletion/mutation in their DNA) increase the Redox Oxidative Species (ROS) concentration8 and cell apoptosis9 which reduce the number of inner ear cells (SG, VS and Hair cells) and, cause a malfunction of the remaining cells.
ABR reflects the way the auditory signal travels from the retrocochlear portion (synapse, SGs, cochlear nerve and cochlear nuclei) (Waves I, III) to the cortex (wave V); if all the structures in the retro-cochlear hearing pathways function correctly, the ABR displays normal amplitude and latency; but damage in even one of the structures causes abnormal ABR latency and/or amplitude, which in turn alter the overall signal shape.
We think that the abnormal ABR of our patient can be explained by the altered functions of residual SGs, which cannot sufficiently amplify the signal to generate an actual ABR wave. The numerous mitochondrial mutations observed in the temporal bone of patients with MELAS10‒11 support the idea that the residual SGs do not function well enough to allow a correct transmission of the impulse, which in turn leads to an abnormal ABR. Arguably, the high ABR variability in MELAS patients reported in the literature2,3,4,6 is due to the stochastic segregation of mitochondria during embryogenesis; the concentration of damaged mitochondria could differ among patients just due to “chance”. In a mitochondrial disease the cellular metabolism is altered (i.e., the ATP is reduced and the concentration of ROS is increased); this could lead to cell death and/or malfunctioning of residual cells.
Unfortunately, mitochondria are segregated in the cells in a random manner so an electrophysiological test such as ABR may not be sensitive enough to measure the inner ear damage. We are in the process of exploring the viability of using different techniques based on microRNAs specific for hair cells and SGs12,13 for monitoring hearing loss progression. Due to their high sensitivity to detect cells death9 and altered function,8 microRNAs may provide a valid alternative to traditional ABR test.
None.
Author declares there are no conflicts of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.