Journal of
eISSN: 2379-6359


Background: The surgery of the posterior fossa is quite complex, the development of neuromodulation allowed many possibilities of treatments for neurological diseases. Auditory brain implant can restore auditory function for people with diseases in cochlear nerves and are not candidates for cochlear implantation such as patients with Neurofibromatosis type 2.
Objectives: To report a case and describe the far lateral surgical approach for brainstem implantation.
Methods: The technique was described and the steps required for the surgery were detailed.
Results: We described in details the surgical technique, how cochlear nucleus can be found, how brain implant must be placed, how to configure auditory brain implant, the intraoperative challenges and possible complications of this surgical approach.
Conclusion: The far lateral surgical approach is a feasible techinique for auditory brainstem implant for patients with neurofibromatosis type 2.
Keywords: auditory brainstem implants, neurofibromatosis 2,hearing disorders, cochlear nucleus, motor, sensory, cephalic, neuroanesthesia, neuroimaging, surgical microscope, brain stem, neuromodulation, posterior cranial fossa, extracochlear electrodes, bipolar coagulation
ABI, auditory brainstem impalnts; EMG, electromyographic activity; NF2, neurofibromatosis type 2
The surgery of the posterior fossa is quite complex by reduced extendable capacity of this compartment, the large number of cranial nerves responsible for essential neurological functions such as motor, sensory, autonomic and visceral cephalic segment and mainly contain structures of the central nervous system that are responsible for maintaining the basic functions for the preservation of life as the heart and respiratory rate and wakefulness. Advances in several technology areas provided greater diagnostic speed, greater availability and better therapeutic surgical technical resources to the development of surgery in the posterior cranial fossa. Among the features available in the operating environment that allowed better surgical outcomes are neuroanesthesia, electrophysiological monitoring, neuroimaging and surgical microscope.1
From a surgical technician view the legacy of Swedish neurosurgeon Herbert Olivecrona started the first five-year period of the twentieth century made possible the scenario that currently live.2 Likewise, implementation of bipolar coagulation, ultrasonography and ultrasonic cleaner made possible microsurgical treatments not considered feasible ago less than thirty years.2The most important advance in this surgical area was the development of neuromodulation. The lead implantation in the brain stem is a form of neuromodulation that can be used to treat various neurological diseases, especially those related to hearing. However, this technique is still not widely used in our field.This paper aims to describe the main anatomical parameters and the far lateral surgical approach for auditory brain stem implant (ABI) in neurofibromatosis type 2 (NF2).
A descriptive study of the surgical approach for placement of auditory brainstem implant (ABI) was performed using the extreme lateral access. The medical records of the sujject was used in this review and detailed all the parameters of the surgical procedure and related devices. Besides, a non-systematic review was performed of the medical literature in PubMed / MedLine, published since 1980 in the Portuguese and English languages, with the following keywords (MeSH): auditory brainstem implant; neurofibromatosis type 2; hearing disorders; cochlear nucleus.
Device (auditory brainstem implant)
The goal of this device (Nucleus 24ABI implant) is to recover and return a certain level of auditory sensation by electrical stimulation of the cochlear nucleus. The system consists of a Nucleus 24ABI implant that need to be used with a external speech processor.3 The Nucleus 24 ABI implant consists of a receiver / transmitter that receives and decodes the electrical impulses from the speech processor and a wire electrode that conducts signals to the surface of the cochlear nucleus in the brainstem. The wire electrodes has 21 electrodes with 0.7 mm diameter and two ground electrodes; an electrode plate set in the transmitter / receiver and a separate ball electrode, the electrode attached to the row. The total number of electrodes is 23. The stimulator implant is functionally identical to 24M Cochlear Nucleus (CI24M). For individuals who need constant MRI, the internal magnet should be replaced during surgery by a non-magnetic titanium plug. (Figures 1 & 2).
The specifications of nucleus 24ABI:
Ethical aspects
Standards of ethics and research committee of the institution have been met.
Detailed Surgical Technique
Marking surgical of the scalp with scalpel blade 11: far extreme right side.
It must be create a dissection section between the proximal portion of the cochlear nerve and choroid plexus, which must be raised/ lifted, along with the cerebellum floc. The cochlear nerve is accompanied medially as he enters in the lateral recess of the fourth ventricle. At this step two protruding structures generally are bent around the brainstem through the tail into the pontobulbar groove: the skull is the protruding structure of the cochlear nucleus, and the flow is pontobulbar body (a set of nerve cells at the bottom of medulla oblongata). Among them a small straight vein is a permanent discovery and an important point of reference. Cut up the tapeworms of the choroid plexus of the fourth ventricle at the entrance, making now the unit can move forward into the lateral recess of the fourth ventricle on the surface of the cochlear nuclei: ventral and dorsal (Figures 3 & 4).
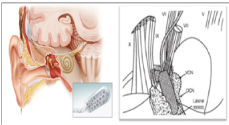
Figure 3 Site of insertion of the electrodes of the brainstem implant 24ABI Nucleusin cochlear nucleus.

Figure 4 Detailed view of the site of insertion of the electrodes of the brainstem implant 24ABI Nucleus in cochlear nucleus.
Placing the electrode wire of the nucleus 24ABI
The electrodes career should be placed on the surface of the brain stem carefully, holding with both microforceps braided network, as the tube on the rear surface of the wire electrodes. The platinum electrodes in the career of “Silastic” (trade name silicone rubber manufactured by Dow Corning) should not be touched by the instruments. Note that the vessels plexus clots and hemostatic agents have to be removed first to obtain a complete optimal contact. The career of electrodes maintains its position within the brainstem grip of a braided network in form of T. The flanks of the network should be wrapped behind the career of electrodes, providing a degree of pressure on the cerebellum. You can get a closer contact with the brainstem, placing a small piece of abdominal fat or gel foam on the back of the career of electrodes, fixing the implant between the cerebellum and brainstem. After electrode placement, place the square screen (located approximately 20mm receiver / stimulator) in the mastoid cavity. This will adhere to the soft tissue around the electrode, and help prevent cerebral spinal fluid to follow along the electrode and accumulate beneath the skin. Verification of correct electrode placement and integrity of the system is completed by recording ABR, electroacoustic response of the brain stem. The position of the electrode can be changed if the ABR responses indicate that the ABI is only partially covering the cochlear nucleus.
It is important to advertisse that once the implant is in contact with the patient, you can not use monopolar cautery. The bipolar electrosurgical instruments may be used, if the cautery electrodes are kept more than 1cm away from any part of the implant or metal electrode. It is aloweed to perform manipulation of the row electrodes using microforceps, needle forceps or pliers on the spare silicone tube attached to the rear surface of the wire or braided network. Neither the surface of the wire electrodes or metal electrode near the wire should be handled by microforceps as there are risks of permanent damage to the device (Figure 5).
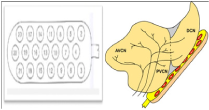
Figure 5 Another view of insertion of the electrodes of the brainstem implant 24ABI Nucleus in cochlearnucleus.
Neural intraoperative monitoring
The procedure used to monitor the status of facial and glossopharyngeal cranial nerves during resection of an acoustic neuroma and subsequent placement of the Nucleus 24ABI involves the continuous recording of live electromyographicactivity (EMG) generated exclusively within the muscles innervated by these cranial nerves. It is desirable, particularly when drawing a large acoustic tumor, providing EMG monitoring of the motor portion of the trigeminal nerve. Also needed is the ability to get potential compound muscle action resulting from direct stimulation of these cranial nerves activated via EMG. In order to obtain a reliable and high sensitivity monitoring of cranial nerve motor, should be avoided as neuromuscular blocking agents during this activity. Table 1 on the following page provides detailed information about the recording location and placement of electrodes for each of the cranial nerves.
Cranial Nerve |
Recording Location / Muscle |
Electrodes and Placement |
V |
Ipsilateral Masseter |
Differential recording, using a pair of needle electrodes placed in 10mm |
VII |
Ipsilateral muscles of facial expression (frontalis, orbicularis oculi, orb. Oris, mentalis, etc.) |
It is recommend two channels, each with differential recordings with a pair of electrodes: Channel 1 - the ipsilateralfrontalis and orbicularis oculi; Channel 2 - in the ipsilateralnasolabial fold and the mentalis muscle. |
IX |
Ipsilateral Soft Palate |
Differential recording using a pair of needle electrodes placed 0.5 to 10mm apart. They should be attached by suture. |
Table 1 Locations and placement of electrodes in neural intraoperative monitoring
Electrical stimulation of the motor cranial nerves
It should be used intracranial electrical stimulation of neural tissue to assist in identification of the cranial nerves associated with the surgical field. Electrical stimulation, pulsed direct current of 100sec duration, can be sent through a bipolar or monopolar probe manual. These stimuli coincide with a clock that enables real-time recording of a muscle action potential associated compound. The level of current to electrical stimulation of the cranial nerves engines may not exceed 2.0mA. The standard power levels for direct neural stimulation vary between 0.1 and 0.5mA. If a monopolar probe is used, we need to put an indifferent electrode near the sterilized surgical field.
Types of electrical responses
Two types of EMG activity are recorded: EMG live and continuous, and muscle compound action potentials associated with cranial neural stimulation directly. The live recording of the EMG provides real-time information of specific cranial neural manipulation and serves as an early indication of the proximity of a particular cranial nerve. This activity consists of a potential activity of a motor mechanism, appearing in a defined channel, associated with the respective cranial nerve. The compound muscle action potentials are typically large amplitude (peak to peak amplitude up to a few thousand microvolts), biphasic and triphasic potentials obtained in real time (not requiring calculation of average signal). The latencies of specific peak of interest, associated with the cranial nerves are: trigeminal 5ms; facial 8 to 10ms; and glossopharyngeal 9ms.
Configuring the ABR intra operative (auditory brainstem evoked potential)
To achieve confirmation of correct placement of the electrodes array during surgery should be recorded the registration of the brainstem-evoked response electrically (ABR) after device placement and prior to closing. During preparation of the patient, the electrodes are placed on the recording surface of the skin and sub-dermal and are connected to a commercial apparatus evoked potential (which may be the same equipment used for monitoring cranial) nerve. The stimulation of Nucleus 24ABI with the use of Cochlear Clinical Programming System (CCPS) or Manual Programming System (MPS), connected to a speech processor and Sprint phone (or HS8 HS9). The stimuli are controlled through the programming Nucleus software. The mediator is triggered by connecting the BNC output MFR5 card on your computer (or 3.5mm MPS) input trigger (trigger) outside. The earpiece and speech are placed inside a sterile kit and the antenna is positioned on the receiving antenna of the implant where the ABR will be engraved processor.
ABR confirmation & electrode placement
The earpiece is placed inside of a sterilized kit and the antenna is installed on the receiving antenna of the implant. Should leave a gap of 2 to 10mm between the transmitting antenna and the implant, and this is achieved with a sterile or removing the magnet from the transmitting antenna and replacing it with the spacer gauze. A combination electrode through the entire wire (E4 to 22 or E2 to 21) is enhanced by increasing the current levels until a response is observed. All combinations of electrodes should be encouraged in both polarities (eg, E2 up to 21 and E21 up to 2) to differentiate an artificial stimulus for a response. Then, a selection of combinations of distal, proximal and lateral electrodes must be chosen, each stimulated in the front and reverse polarity, to establish the optimal placement of the Nucleus 24ABI. The surgeon needs to commit a carefully manipulation of the wire electrode to find an ideal position with maximum wire responses. Should avoid a comprehensive repositioning due to increased risk of damage to the surface of the cochlear nucleus neurons and surrounding tissue of the brain stem. Typically, it is observed responses of 2 or 3 wave, although sometimes only one wave appears clearly. The major late potentials, more than 4msec are an indication of stimulation of other cranial nerves. Two appendices, a record of intraoperative ABR and a diagram of the electrodes are included. While stimulating wire electrodes in an attempt to confirm the electrode placement, should be continued monitoring of cranial nerves V, VII and IX. The purpose of this approach is to detect and identify the non-auditory stimuli associated carelessly placing the Nucleus ABI and stimulation of other cranial nerve. For example, in the implant device activation you could identified the compound muscle action potential with a latency of 9 msec peak in the EMG channel tripped, associated cranial nerve IX, is probably indicated that the electrode is in the vicinity of the glossopharyngeal nerve.
It is important to note that by driving volume, this response may appear in the saved channel associated with ABR. A real ABR exists when appears an answer in only one channel and not the at other channels of the monitoring cranial nerve and this answer needs to be a correct latency and amplitude. In summary, the presence of ABR confirmed to be correct by evaluating the amplitude peaks, the appropriate response latency, and distribution of recording - that is, one can see coincident responses in any of the other channel monitoring cranial nerve. This situation is conducted at Nucleus Stimulation 24ABI with Sprint speech processor (by turning it the handset or HS8 HS9), using the Clinical Programming Manual or Cochlear System (MPS or CPS) and the programming software (Figure 6).
ctivation of auditory brainstem implant nucleus 24 ABI postoperatively
The activation process should be scheduled about six weeks after surgery. Typically, several sessions are necessary mapping until you get a better fit for the patient. The speech processor adjustment must be made in the third month, sixth month and one year after surgery, and thereafter every year (or more often if the patient needs). It should provide extra training to the patient, if necessary. During follow-up, tests psychophysical responses shall be made in addition to a performance evaluation.
Patient, 29years-old, female, diagnosed with type 2 neurofibromatosis since childhood. Presents deafness had slowly progressive, bilateral evolution, progressive visual impairment and motor deficits (mainly the lower limbs). Still has a left facial palsy, House-Brackman grade 2 (sequel of prior surgery / tumor). She had been submitted to several surgical approaches for removal prior tumor lesions of the spine, intracranial, symptomatically. Preoperatively the patient already had functional impairment of cranial nerves III, VI, VII. VIII. Was assessed from an audiological perspective, with no hearing benefits with conventional hearing aids. Patient, even with visual deficits, makes use of lip reading and auditory support. The cochlear implant was discarded because it presents several injuries, bilateral, in the internal auditory canal, the cerebellopontine angle and posterior fossa. Expectations regarding the performance of the auditory brainstem implant were worked preoperatively.
Neurofibromatosis type 2 (NF2)
It is known as central neurofibromatosis or bilateral acoustic neurofibromatosis. The vesticulares bilateral schwannomas are the hallmark of the disease and invariably cause hearing loss.4 In 1822, the NF2 has been described in a young baker who presented to the Scottish surgeon James Wishart with bilateral progressive deafness. He underwent neurological surgery for removal of intracranial tumors and succumbed days after the procedure due to septicemia. At autopsy multiple tumors were identified in the dura, the brain and eighth cranial nerves bilaterally.5 Currently it is known that NF type 2 is the most phakomatosis rare, autosomal dominant, with an incidence of 1:40,000 live births.6
NF2 is associated with a defect in “merlin / schwannomin” gene on chromosome 22.7 The current diagnosis of NF2 was facilitated by more specific criteria.8
Complications and difficulties of far lateral right surgical aprroach
From the surgical point of view of the technical difficulties to access are associated with existence of large anatomical variation between the vascular and nerve structures in access which favors the occurrence of complications.9 In addition to excessive bone removal for retrosigmoid approach can both increase the risk of CSF leak and cause damage to venous structures that are catastrophic.10,11 The mortality rate for access to the base of the skull posterior fossa may reach 1.3% of cases.12 Among other complications for surgical implant electrodes in the brain stem are the main cerebrospinal fluid leak, facial paralysis, meningitis, wound infection, contusional cerebellar lesion, dysphagia.12,13 In large case series of major complications (eg, stroke, seizures, persistent cerebellar dysfunction) can reach about 4% and smaller as mentioned above can reach 12% of cases.13 Patients with NF2 or other neurological diseases or complications are similar between adults and children.13
The far lateral surgical approach is a feasible techinique for auditory brainstem implant for patients with neurofibromatosis type 2. After the tumor is removed the location of the cochlear nucleus must be identified to place the electrode wire of the ABI.
We would like to thank the patient and the patient family. Thanks to everyone who helped us in this procedure, and helped us directly or indirectly to perform this paper, and all people of our department.
Author declareas there are no conflicts of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.