Journal of
eISSN: 2373-437X


Research Article Volume 10 Issue 1
Bacteriology, Virology Laboratory, Arrazi Hospital, University Hospital Mohammed VI of Marrakech, Faculty of Medicine and Pharmacy of Marrakech, University of CADI AYYAD of Marrakech, Morocco
Correspondence: Hajar Skali, CHU Mohammed VI BP2360 Principal Av. Ibn Sina, Marrakesh, Morocco, Tel +212679396651
Received: January 23, 2022 | Published: January 28, 2022
Citation: Skali H, Lazrak FZ, Hanchi AL, et al. Urinary tract infections in urology: highlight on the epidemiological and bacteriological profile. J Microbiol Exp. 2022;10(1):18-22. DOI: 10.15406/jmen.2022.10.00347
Background: Urinary tract infection is one of the commonest infection occurring in all age groups and one of the most frequent in hospital practice, particularly in Urology department where the use of invasive urological maneuvers is frequent. Knowing the common isolated uropathogens and their antimicrobial susceptibility is beneficial in planning diagnostic and therapeutic guidelines. This work aims to evaluate the prevalence of Urinary tract infection in patients hospitalized in Urology department of the University Hospital Mohamed VI and to highlight its epidemiological and bacteriological characteristics.
Methods and Material: This retrospective descriptive study was carried out at the Microbiology laboratory of the University hospital Mohamed VI of Marrakech, over a period of 24 months (2018 –2019), including all urinary tract infections documented by a positive cytobacteriological examination of urine obtained from the Urology Department.
Results: Nine hundred and fifty-two samples were analyzed. The positivity rate to bacteria was at 18.5%. The mean age was 56 years. The male gender was predominant with a sex-ratio (M/F) at 2. Clinical urinary signs were dominated by burns during urination (80%), followed by pollakiuria (64%) and dysuria (49%). Nosocomial Urinary tract infection represented 28% of cases of Urinary tract infection hospitalized in Urology department. The strains isolated were mainly represented by Enterobacteriaceae (58%), dominated by Escherichia Coli (27%,), followed by Klebsiella pneumoniae (19%) and Enterobacter cloacae (8%). Enterobacteriaceae were resistant to third-generation cephalosporins in 45%. Twenty-four percent of isolates had decreased susceptibility to carbapenems. The resistance mechanisms highlighted were mainly the production of the Extended Spectrum Betalactamase which was detected in 42% of isolated Enterobacteriaceae, represented mainly by Klebsiella pneumoniae (53%), followed by Escherichia coli (26%) and Enterobacter cloacae (19%).
Conclusion: It is important to rationalize the use of antibiotics that have good antibacterial activity through increased awareness of stakeholders and the establishment of appropriate consultation and regulatory frameworks.
Keywords: urinary tract infection, antibiotic resistance, nosocomial, multidrug-resistance organism, urology, nosocomial
Urinary tract infection (UTI) is one of the commonest infection occurring in all age groups worldwide, with an estimated annual global incidence of at least 250 million.1 UTI a term applied to variety of clinical conditions, ranging from asymptomatic bacteriuria to severe infection of kidney with resultant sepsis. Acute pyelonephritis, renal or perirenal abscess are also included as UTI and considered major infections. Other types of infection are infections of the male accessory glands such as acute orchitis or prostatitis. It also includes UTI in patients with urinary cathetersis.2 From microbiological perspective, it exists when pathological microorganisms are detected in urine, urethra, bladder, kidney or prostrate. Many microorganisms can infect the urinary tract, but by far the most common agents are gram- negative bacilli.3,4 UTI is one of the most frequent infections in hospital practice. It is said ‘nosocomial’ or ‘nosocomially acquired’ (NUTI or NAUTI) when it is acquired in any healthcare institution or, more generally, when it is related to patient management.5 NUTI represents more than 30% of nosocomial infections. It’s a real public health problem, being costly to patients and health care funding agencies, and controversial with regard to management strategies. Increase in costs is due to an increase in medical requirements and a more extended hospital stay.6 Patients admitted to Urology Department have an increased risk of developing NUTI with a percentage rising to 60–70% of all nosocomial infections. They frequently undergo some type of surgical procedure during hospitalization, and a high percentage are carriers of a urinary catheter both before and during admission.7,8 Due to the multiple prescription of antibiotics and the lack of standardization of antibiotic susceptibility test, resistance to commonly microorganisms responsible for UTI is increasing year by year with a rise in multidrug-resistant bacteria. Knowing the common isolated uropathogens and their antimicrobial susceptibility is beneficial in planning diagnostic and therapeutic guidelines.9,10 This study aims to evaluate the prevalence of UTI in patients hospitalized in Urology department of the University Hospital Mohamed VI and to highlight its epidemiological and bacteriological characteristics.
This retrospective descriptive study was carried out at the Microbiology laboratory of the University hospital Mohamed VI of Marrakech, over a period of 24 months (January 2018 –December 2019), including all urinary tract infections documented by a positive cytobacteriological examination of urine (CBEU) obtained from the Urology Department.
The CBEU was carried out in accordance with the recommendations of the medical microbiology referential which included culture, quantification of leukocytes and erythrocytes, identification and quantification of microorganism(s) involved and the study of antibiotic susceptibility.11 Qualitative and quantitative cytological analysis was performed for each specimen received using microscopic methods or automated urine analyser (Sysmex UF-1000i®). The identification of microorganism was according to morphological, cultural, biochemical and antigenic characters, using a manual identification by API® 20 E (Biomerieux, France) or automated identification system by BD Phoenix®.
The antibiotic susceptibility test was performed by using disc diffusion or microdilution method (BD Phoenix®) and was carried out according to the standards of the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
The biological diagnosis of UTI was carried out on a leucocyturia ≥104/ ml associated with a significant bacteriuria which was interpreted according to the bacterial species involved and the sex of the patient.12 We recorded for each sample: age, sex, risk factors, history of hospitalization, clinical symptoms, isolated bacteria and its susceptibility to antibiotics. The nosocomial character was retained on a positive CBEU after 48 h hospitalization.
Statistical analysis and data entry was carried out by Microsoft office Excel 2007. The frequency of standard descriptive statistics such as mean and standard deviation were used to summarize patient characteristics.
General epidemiology of UTI in urology
Over a period of 2 years, 952 samples obtained from the Urology department were sent to the Microbiology Laboratory for cytobacteriological study. Among these samples, 176 cases were having the criteria for UTI with a prevalence rate at 18.5%. The mean age was 56 years with extremes ranging from 19 to 93 years. The most affected age range group was the group of patients older than 60 years. The male gender was predominant with a sex-ratio (M/F) at 2 (Figure 1).
Clinical urinary signs were dominated by burns during urination (80%), followed by pollakiuria (64%), dysuria (49%), lower back pain (45%), macroscopic hematuria (20%), fever (13%), pain in the suprapubic region (10%) and pyuria (4%) (Figure 2).
Patients admitted to a Urology department received an antibiotic prophylaxis maintaining sterility in the surgical field and in the hospitalization unit, and removal of urinary catheters. The prophylaxis molecule used was cephalosporin of first generation. Otherwise, treat and sterilize the urine before any gesture was the rule. Twenty-eight percentage of cases of UTI hospitalized in Urology department were nosocomial with multidrug-resistant bacteria.
Distribution of uropathogenic species isolated in urology
The bacteriological profile was dominated by Gram negative bacteria in 72 % of cases (n=176). The strains isolated were mainly represented by Enterobacteriaceae (58%, n=176), dominated by E. Coli (27%,), followed by Klebsiella pneumoniae and Enterobacter cloacae found respectively in 19% and 8% of cases. Other types of Enterobacteriaceae were isolated in 4%. The non-fermentative gram-negative bacteria were isolated in 14% (n=176) with Acinetobacter baumannii in 8% and Pseudomonas aeuroginosa in 6% of cases. Candida spp strains were found in 12% (Figure 3).
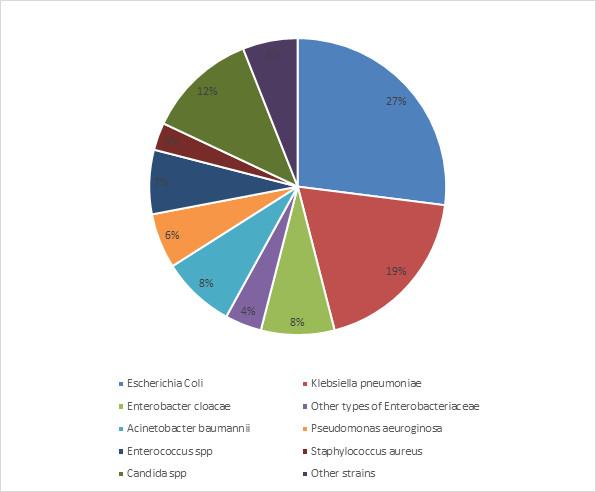
Figure 3 Distribution of bacteria isolated from urines samples obtained from the Urology department of the University Hospital of Marrakech (n=176).
Study of the antibiotic resistance profile of uropathogenic Enterobacteriaceae strains
Enterobacteriaceae Strains were resistant to amoxicillin in 90%, to amoxicillin-clavulanic acid in 66%, and to third-generation cephalosporins in 45%. Twenty-four percent of isolates had decreased susceptibility to carbapenems, with resistance to ertapenem in 20% and to imipenem in 4% of the isolates (Figure 4).
In Enterobacteriaceae, the resistance rate of K. pneumoniae was high compared to that of E. coli and Enterobacter cloacae for the majority of the antibiotics used (Figure 5).
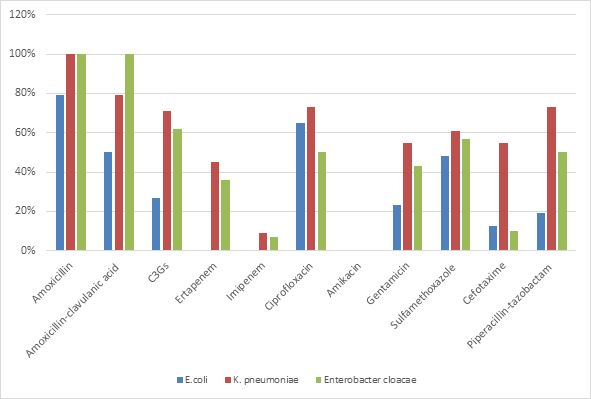
Figure 5 Comparison of antibiotic resistance profile of the most common Enterobacteriaceae strains (n=95)
Resistance to third-generation cephalosporins by production of Extended Spectrum Betalactamase in 42% (n=101) of isolated Enterobacteriaceae, represented mainly by Klebsiella pneumoniae (53%), followed by Escherichia coli (26%) and Enterobacter cloacae (19%). Fifty-one percentage of these Extended Spectrum Betalactamase -producing Enterobacteriaceae showed decreased susceptibility to carbapenems. Multidrung-resistant bacteria strains accounted for 28% (n=176), and were mainly represented by Enterobacteriaceae in 76% (n=49) followed by Acinetobacter baumannii in 20% and Pseudomonas aeuroginosa in 4% of cases.
Evolution of the antibiotic resistance of Enterobacteria uropathogens between 2018-2019
The monitoring of the evolution of antibiotic resistance in the most frequent uropathogenic isolates in Urology (E. coli and K. pneumoniae) showed a significant increase (table 1). In E. Coli, a gradual increase in resistance to main antibiotics was found, exceeding the 60% in 2019 for AMP, AMC and CIP. In K. pneumoniae showed very high percentages of antibiotic resistance exceeding 60% for the majority of antibiotics and unlike E. coli, the evolution of resistance to carbapenems was significant during the study period.
ATB |
E.coli % R |
E.coli % R |
K.p % R |
K.p % R |
Year |
2018 |
2019 |
2018 |
2019 |
Third-generation cephalosporins |
26.9 |
27 |
74 |
65 |
Amoxicillin |
62 |
85 |
100 |
100 |
Amoxicillin-clavulanic acid |
30 |
62 |
86 |
80 |
Piperacillin-tazobactam |
15 |
23 |
78 |
63 |
Ertapenem |
0 |
0 |
36 |
54 |
Imipenem |
0 |
0 |
35 |
38.5 |
Ciprofloxacin |
62 |
65 |
86 |
61.5 |
Amikacin |
0 |
0 |
0 |
0 |
Gentamicin |
27 |
19 |
50 |
61.5 |
Sulfamethoxazole |
48 |
46 |
57 |
61.5 |
Table 1 Evolution of the antibiotic resistance of E. coli and K. pneumoniae between 2018-2019
Abbreviations: ATB, antibiotic; E. coli, Escherichia coli; Kp, Klebsiella pneumoniae
Urinary tract infection has been one of the most common conditions seeking for hospital visit and treatment in clinical practice. It has been studied extensively by many people. The UTI clinical profile can range from simple cases such as cystitis to severe cases such as uroseptic shock. Empirical antibiotic treatment is usually the first treatment to be administered to patients with UTI. Therefore, it is essential to be aware of the epidemiological data for an appropriate initial treatment.13,14 This study highlights the problem of UTI in our hospital, particularly in the urology department, where the use of invasive urological maneuvers is frequent.
In our study, the prevalence of UTI was in order of 18,5% which was close to the results of the study conducted by N. Subedi et al. with culture positivity at 17.4%.10 Other studies showed higher prevalence of 31% and 24.5%.15-17 In Mansour et al. study,18 culture was positive in 8.7%. This variability in UTI prevalence could be explained by a number of risk factors: anterior antibiotic treatment, surgical or endoscopic invasive intervention, the duration of hospital stay.
In literature, the female predominance found in UTI in the urological patient is related to the anatomical characteristics of women: shortness of the urethra, proximity of genital and anal orifices, inadequate hygiene practices, sexual intercourse and pregnancy.10,16,18 In our study, the results showed male predominance (sex-ratio at 2) which could be explained by the choice of the department of Urology where most of the patients hospitalized were male. Similar results were also reported in other study.17
The mean age was 56 years and the most affected age range was the group of patients older than 60 years. Similar data in relation to the mean age was reported by other study.17 This could be explained by the main reason for hospitalization in the urology department is bladder tumor which occurs in older population. Lower mean age was described in other works.10,16
Clinical signs were dominated by burns during urination (80%), followed by pollakiuria (64%), dysuria (49%). UTI symptoms are very important factors leading to the diagnosis. Physicians must be aware of the atypical symptoms because when a UTI symptom is not recognized, treatment is delayed and it has significant impact on mortality and health care costs and quality of life of patients.19
Nosocomial UTI represented 28% of cases of UTI hospitalized in Urology department. Other foreign works reported lower rate of NUTI which could be due to differences in the definition criteria of NUTI, to protocols installed and awareness among medical staff, nurses, patients and their relatives.20,21
Escherichia coli and Klebsiella pneumoniae were the most predominant uropathogenic bacteria which was in accordance to several national and international series.10,17,18,20 These bacteria are usually commensals of the human digestive tract. The poor hygiene and asepsis conditions make these bacteria often involved in various human pathologies, including UTI.
In our work, we studied the antibiotic susceptibility of Enterobacteriaceae strains isolated and it had revealed resistance to amoxicillin-clavulanic acid in 66% of cases, to third-generation cephalosporins in 45%, to fluoroquinolones in 62%. These resistance rates were comparable to those of Ferjani et al. in Tunisia.22 In our study, Klebsiella pneumoniae was found to be more resistant to tested antibiotics than other commonly associated organisms which was similar to literature data.22,23
In the present study, the resistance mechanisms highlighted were mainly the production of the Extended Spectrum Betalactamase which was detected in 42% of isolated Enterobacteriaceae, represented mainly by Klebsiella pneumoniae (53%), followed by Escherichia coli (26%) and Enterobacter cloacae (19%). This results were comparable to other works,24-28 and were higher than those reported in other studies.29,30 The causes associated with high levels of Extended Spectrum Betalactamase could be the self-medication with excessive consumption of antibiotics without any medical prescription. This excessive use is often based on a bundle of clinical argument, without bacteriological examination. This practice is a serious problem in developing countries such as ours, and contribute to the selection of multi-resistant strains.
Twenty-four percent of isolates had decreased susceptibility to carbapenems, with resistance to ertapenem in 20% and to imipenem in 4% of the isolates. That could be explained by the use of carbapenems which is becoming more and more frequent in view of the increased resistance to third-generation cephalosporins in bacteria responsible for UTI in patients in urology, leading to the emergence of carbapenem-resistant strains. The rational use of these so-called last resort molecules is mandatory in order to avoid the emergence of carbapenemase-producing strains.
The monitoring of the evolution of antibiotic resistance of E. coli and K. pneumoniae showed a significant increase, which reflects the intensive prior use of antibiotics in medical settings.
Urinary tract infection is a common problem worldwide, its recognition, proper diagnosis with urine culture and starting appropriate antibiotics according to the culture report plays a major role in preventing complicated UTI. It is one of the most frequent infections in hospital practice, particularly in Urology department where the use of invasive urological maneuvers is frequent. Escherichia coli is the most common organism isolated in most of the hospitals. Antibiotic sensitivity pattern in a particular area will give an idea to clinicians regarding empirical treatment of UTI before the availability of laboratory reports. The increase of the resistance of Escherichia coli and Klebsiella pneumoniae observed in this study reflects the intensive prior use of antibiotics in medical settings. The widespread use of third-generation cephalosporins molecules complicates the therapeutic decision and forces the clinician to prescribe a broad spectrum antibiotherapy such as carbapenems which had led to the emergence of strains with decreased sensitivity to carbapenems. We should encourage the rational and controlled use of antibiotics through increased awareness of stakeholders and the establishment of appropriate consultation and regulatory frameworks.
None.
Authors declare that there is no conflict of interest.

©2022 Skali, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.