Journal of
eISSN: 2373-437X


Research Article Volume 8 Issue 3
Taras Shevchenko National University of Kyiv, Ukraine
Correspondence: Iryna Akulenko, ESC “Institute of biology and medicine”, Taras Shevchenko National University of Kyiv, 03127, Kyiv, Hlushkova Avenue, 2, Ukraine, Tel +380974804114
Received: June 11, 2020 | Published: June 30, 2020
Citation: Akulenko I, Skovorodka M, Serhiichuk T, et al. The oxalate-degrading activity of Lactobacillus spp. isolated from different sources as the potential probiotic modulators for oxalate homeostasis. J Microbiol Exp. 2020;8(3):118-123. DOI: 10.15406/jmen.2020.08.00295
Background: Currently, diseases of the urinary system are observed in 3.5–4% of the world's population. According to WHO, the number of people suffering from this pathology doubles every 7–10 years. To date, hyperoxaluria is considered as the main risk factor for the formation of oxalate-calcium stones, which account for 75% of all kidney stones. One of the main causes of hyperoxaluria is a decrease in the number of microorganisms capable of degrading oxalates, which occurs due to the disruption of the intestinal microbiota. Oxalate-degrading bacteria include the genera Lactobacillus, Bifidobacterium, Oxalobacter formigenes etc. Searching of probiotic strains with high oxalate-degrading activity have become one of the priorities from the context of research. The aim of the present study was to isolate Lactobacillus spp from different sources and to determine their ability to degrade oxalate.
Methods: A total of 23 Lactobacillus spp. from food of animal and vegetable origin were isolated with selective MRS Broth medium and further cultured on MRS Agar or Oxalate Medium with 5 g/l sodium oxalate. ANAERO test23 was used to assess the species affiliation. Oxalate-degrading activity (ODA) was measured by redox titration with KMnO4.
Results: Only 7 species of isolated bacteria out of 23 showed the ability to grow on the oxalate-containing culture medium. According to the morphological and physiological-biochemical characteristics, these bacteria belonged to the genus Lactobacillus: L. nagelii – 2 spp, L. rhamnosus – 2 spp., L. frumenti - 1 spp, L. plantarum – 1 spp, L. acidophilu – 1 spp. The most active metabolizers of oxalate on Oxalate Medium were L. plantarum S3 – 42%; L. acidophilus S5 – 38%, and L. nagelii Z2 – 35%; the worst results were shown by L. rhamnosus K7 and L. nagelii S12 – both metabolized only 7% of sodium oxalate.
Conclusions: The redoximetric titration with KMnO4 was adopted to evaluate the ODA of bacteria in culture media. Lactobacillus spp. isolated from different sources differs according to the level of ODA. Three promising Lactobacillus species were selected for further estimation of probiotic profile.
Keywords: oxalate, urolithiasis, hyperoxaluria, oxalate-degrading bacteria, probiotics
Oxalate is an anion of dicarboxylic oxalic acid (H2C2O4). It is quite common in nature. Some foods, such as vegetables and cereals contain large amounts of oxalic acid and their consumption can lead to a significant increase in oxalate excretion.1 Oxalic acid is toxic at higher levels and its accumulation in the body can lead to various diseases of the urinary system such as hyperoxaluria.
Oxalates can be absorbed in the urinary tract and excreted in the urine. Alternatively, intestinal oxalate may combine with calcium to form insoluble CaOx, which is excreted with feces. In addition, its level can be reduced by gastrointestinal tract microorganisms. The relative amounts of calcium and oxalate are important factors influencing the rate of absorption and excretion of this compound.2 Bacteria of the GIT (gastrointestinal tract) decompose many dietary substances that cannot be digested by humans, including oxalate.3
Degradation of oxalate by bacteria occurs through the aerobic and anaerobic pathways. During aerobic growth, oxalate is metabolized to CO2 and formate, then to formate dehydrogenase which oxidizes the latter compound. Anaerobic bacteria, such as those found in the GIT, are not able to oxidize formate, so it accumulates as the main end product of oxalate catabolism.4
Conditionally bacteria can be divided into the "broad-profile oxalotrophs" (generalists) which are not completely dependent on oxalate as an energy source and can ferment many other substrates in addition to Lactobacillus, Bifidobacterium, Enterococcus, Bacillus etc, and "narrow-profile oxalotrophs" (specialists) which use oxalate as the sole or main energy source and carbon (Oxalobacter formigenes).5
O. formigenes is obligate anaerobic bacterium and powerful oxalate destructor. So far, O. formigenes use as the probiotic has some difficulties, probably because of challenges in the preparation of O. formigenes.6 The search for O. formigenes strain which is able to persist in the absence of oxalate, to be aerotolerant, and to survive for long periods when freeze-dried or mixed with yogurt;7 or alternative bacteria species with oxalate degrading activity (ODA) looks like the reliable options.There are several studies that validates the ODA of different Lactobacillus species. Studies by Turroni et al.8 found a number of Lactobacillus spp. that are able to degrade oxalate. Frc and Oxc genes were found in all isolates of L. acidophilus and L. gasseri, which degraded more than 50% of oxalate.
Thereby, the aim of this study was to isolate bacteria from different sources and determine their ability to degrade oxalate in culture media with oxalate.
Sources of bacteria
A total of 23 Lactobacillus spp were isolated from food of animal and vegetable origin: spinach, tomato, sour cream, sour milk, cream, cottage cheese and sourdough purchased on local Kyiv markets, Ukraine.
Isolations of bacteria
Lactobacillus spp were isolated with selective MRS Broth medium with next composition (g/l): proteose peptone–10, HM peptone B–10, yeast extract–5, dextrose (glucose)–20, polysorbate 80 (tween 80)–1, ammonium citrate–2, sodium acetate–5, magnesium sulphate–0.1, manganese sulphate–0.05, dipotassium hydrogen phosphate–2 («HiMedia Laboratories», India). A small amount of product, previously homogenized to liquid state, was added into the test tubes with MRS Broth and cultured under anaerobic conditions in a thermostat at 37oC for 48 h. After that the cultures were seeded on both MRS Agar («HiMedia Laboratories», India) and Oxalate Medium (g/l):9 K2HPO4–0.25, KH2PO4–0.25, (NH4)2SO4–0.5, MgSO4·7H2O–0.025, CH3COON–0.82, yeast extract–1.0, rezazurin–0,001, Na2CO3–4, L-cystein-HCl–0,5, Trace element solution SL-10–1 ml (mix/L: HCl (25%; 7.7 M)–10.00 ml, FeCl2 x 4H2O–1.50 g, ZnCl2–70.00 mg, MnCl2 x 4H2O–100.00 mg, H3BO3–6.00 mg, CoCl2 x 6H2O–190.00 mg, CuCl2 x 2H2O–2.00 mg, NiCl2 x 6H2O–24.00 mg, Na2MoO4 x 2H2O–36.00 mg; Na2C2O4–5 mg) to test the ability to grow on a solid medium containing sodium oxalate as the sole source of carbon and energy. The each newly isolated species was reseeded 5 times on Oxalate Medium to analyses their viability and sustainability.
Identification
Cell morphology was examined by Gram-stained smear microscopy (Gram Staining Kit, Ukraine).
The spectrum of carbohydrate fermentation was investigated by ANAEROtest 23 («Erba Lachema», Czech Republic). In brief, from pure 48-hour culture, a suspension was prepared in suspension medium for ANAERO test 23, density 3.0 according to the McFarland standard (9×108 CFU/ml). Then 0.15 ml of suspension in all wells of the corresponding 3 rows of the plate was made. These wells contained certain carbohydrate substrates: glucose, maltose, fructose, galactose, lactose, melecytosis, sucrose, trehalose, rhamnose, esculin, mannose, raffinose, cellobiose, xylose, arabinose. This kit also contained a test for the recovery of nitrates. The control was a medium that did not contain a hydrocarbon substrate. The results were recorded after 48 hours. Changing the color of the medium from purple to yellow meant a positive reaction, except for the test for esculin hydrolysis and nitrate reduction. Cleavage of esculin gives a black color. And for the test for nitrates in the appropriate well was added Griess test, a positive result is the appearance of red color. The ABIS Identification database was used to interpret the obtained results.
Growth curves
Growth curves were constructed to study bacterial growth on MRS Broth and Oxalate Medium with 5 g/l of sodium oxalate. MRS Broth without sodium oxalate was used as the control medium. Pure culture of isolated Lactobacillus spp was seeded in oxalate-containing MRS Broth and Oxalate Medium or control medium with initial concentration 108 CFU/ml adjusted by Densi-La-Meter II. Before the cultivation we measured initial optical density (OD) for each bacterial suspension. Then it was cultured in a thermostat at 37oC for 72 h. The absorbance was determined every hour on fluorescence spectrometer LS 55 («Perkin Elmer», USA) with wavelength λ=540 nm. For each bacterium we measured the beginning of exponential and stationary phases, samples selected at these two points then centrifuged at 3000 g for 15 minutes to discharge the bacterial biomass. Supernatants used to determine the ODA of Lactobacillus spp in Oxalate Medium and MRS Broth + oxalate.
Determination of oxalate-degrading activity
The redoximetric titration with KMnO4 was adopted to evaluate the ODA of bacteria in culture media.10,11
An aliquot of 10 ml test solution and 10 ml Oxalate Medium (control) or MRS Broth + oxalate (control) was centrifuged 3000 g for 15 min, T room. Supernatant 10 ml was transferred to a 50 ml beaker. Calcium oxalate was precipitated with the addition of 10 ml of 0.4 M Ca(NO3)2. The precipitate formed is filtered out with double paper filters with a low filtration rate (80 g/m2). The filtrate was discarded. Precipitated calcium oxalate was dissolved with 25 ml of H2SO4 (1:4). The acidified 10 ml calcium oxalate solution mixed with 20 ml deionized water was heated to 80°C prior to titration. Immediately, 10 ml of H2SO4 (1:4) solution was added and titrated with KMnO4 (0.02 N) solution until a pink color persists for 30 seconds. The results were expressed in % degradation of sodium oxalate.
Data Analysis
All statistical analyses were performed using Origin 9 (OriginLab, Northampton, MA) software. All data were presented as numbers and percentages. The statistical correlation between the percentages of ODA and growth rate was evaluated using the Pearson index. The index could range between -1 and +1, were -1 means negative linear correlation, +1 – positive direct correlation. If the index is between 0 and 0.3 – correlation is weak, 0.3 and 0.7 – correlation is moderate. If the index is higher than 0.7 correlations is strong.
Isolation of oxalate-degrading bacteria from different sources
From 23 species of bacteria isolated from different food of animal and vegetable origin only 7 saved their ability to grow on media that contain oxalate (Table 1).
Source |
Number of isolates on MRS agar |
Number of isolates on Oxalate Medium |
Number of isolates after 5 times reseeding on Oxalate Medium |
Spinach |
1 |
1 |
0 |
Tomato |
1 |
0 |
0 |
Sour cream |
5 |
3 |
3 |
Sour milk |
6 |
4 |
3 |
Cream |
2 |
0 |
0 |
Cottage cheese |
4 |
2 |
0 |
Sourdough |
4 |
3 |
1 |
Total |
23: spinach, tomato, sour cream, sour milk, |
13: spinach, sour cream, sour milk, cottage |
7: sour cream, sour milk, |
Table 1 Quantity of isolated bacteria from different sources
Species identification of isolated oxalate-degrading bacteria from different sources
According to morphological-cultural and physiological-biochemical characteristics, isolated bacteria belonged to the genus Lactobacillus. All newly isolated bacteria were rod-shaped, Gram-positive, did not form gas when grown on glucose medium, all were catalase-negative.
The spectrum of carbohydrate fermentation was investigated in selected bacteria. But Lactobacillus did not differ much in these properties; the only difference was in the decomposition of melezitose. Fermentation of this carbohydrate is not a marker and may vary in strains of one species of Lactobacillus. This fact proved that these microorganisms can be attributed to one genus.
The spectrum of carbohydrate fermentation was investigated by ANAERO test 23. All studied species fermented glucose, maltose, fructose, galactose, lactose, sucrose, trehalose, rhamnose, mannose, cellobiose, arabinose. None used xylose and raffinose. It should also be noted that no culture was able to produce indole and was not able to decompose urea, as the urease enzyme was absent.
Also one of the signs confirming the identity of selected bacteria to the genus Lactobacillus is decompose аesсulin. The results showed that all the studied species are able to decompose aesculin (Table 2).
№ |
Source |
Species |
Identity, % |
Z2 |
Sourdough |
Lactobacillus nagelii |
99 |
K7 |
Sour milk |
Lactobacillus rhamnosus |
91.1 |
K8 |
Sour milk |
Lactobacillus rhamnosus |
90 |
K9 |
Sour milk |
Lactobacillus frumenti |
86.5 |
S3 |
Sour cream |
Lactobacillus plantarum |
98 |
S5 |
Sour cream |
Lactobacillus acidophilus |
96.3 |
S12 |
Sour cream |
Lactobacillus nagelii |
91.4 |
Table 2 The results of physiological and biochemical identification by ANAERO test 23
The growth curve plot of isolated oxalate-degrading bacteria dependently on oxalate presence in culture medium
We studied the duration of the growth phases of isolated bacteria on the different medium: MRS Broth, MRS-broth with sodium oxalate and Oxalate Medium (which contained sodium oxalate as the sole source of energy and carbon). The exponential growth phase was similar for all bacteria in MRS Broth, 4±1 hours after the start of cultivation. On MRS Broth with sodium oxalate, the exponential phase began later, in 20±10 hours. It is possible that the presence of oxalate initially inhibits growth and the bacteria have to adapt longer (Figure 1). But it is important to note that L. plantarum S3 (Figure 1E) in MRS Broth with sodium oxalate start to grow immediately after beginning of cultivation.
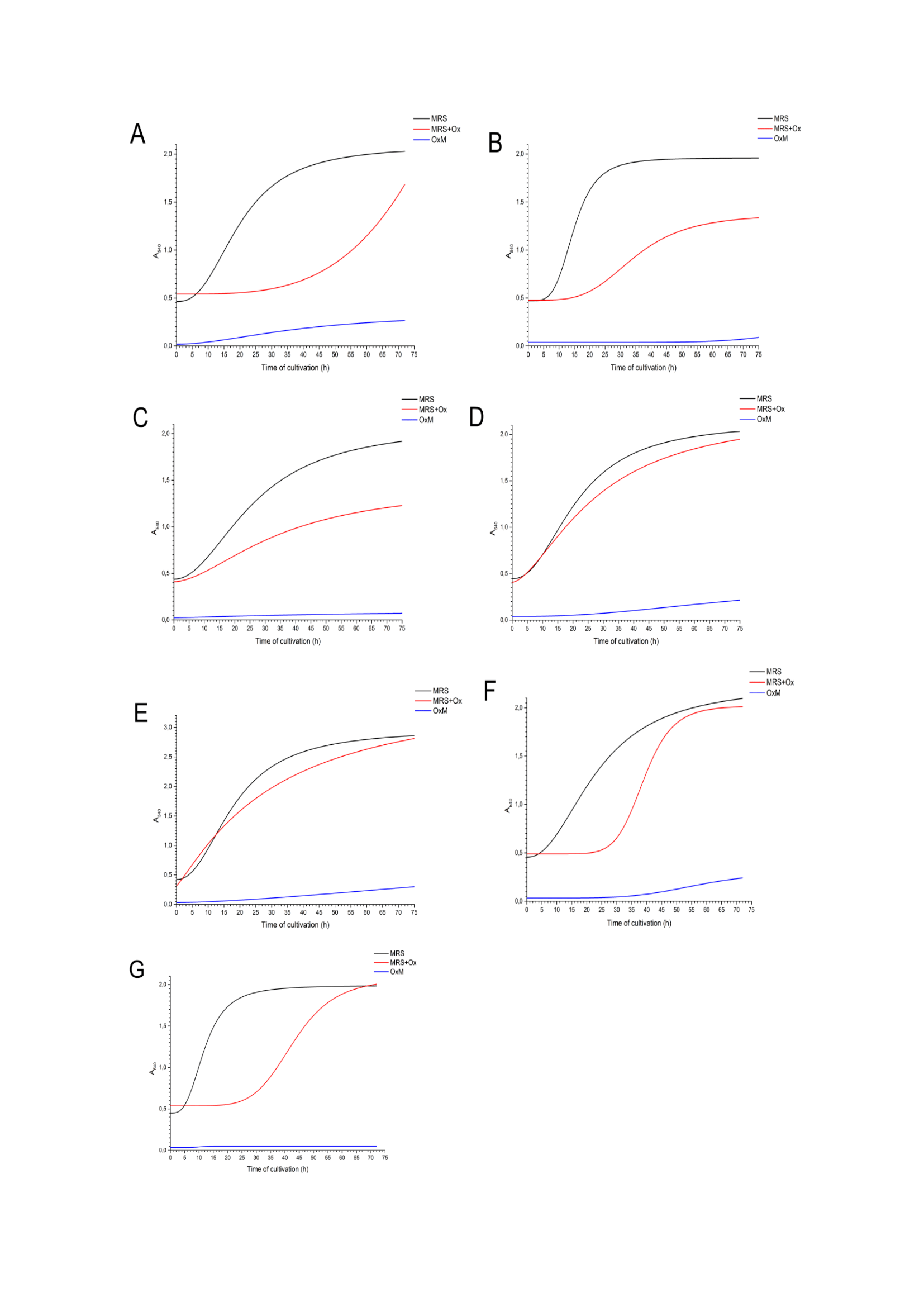
Figure 1 The growth curves of isolated oxalate-depredating bacteria dependently on oxalate presence in different culture medium (MRS Broth, MRS Broth+oxalate, Oxalate Medium): (A) L. nagelii Z2, (B) L. rhamnosus K7, (C) L. rhamnosus K8, (D) L. frumenti K9, (E) L. plantarum S3, (F) L. acidophilus S5, (G) L. nagelii S12.
The level of oxalate-degrading activity of isolated bacteria
In the following stage, the oxalate degrading activity of 7 selected strains of Lactobacillus were tested: L. nagelii Z2, L. rhamnosus K7, L. rhamnosus K8, L. frumenti K9, L. plantarum S3, L. acidophilus S5, L. nagelii S12 on MRS Broth with sodium oxalate and on Oxalate Medium. The aim was to test the ability of bacteria to degrade oxalate not only in the optimal media, but also in media in which oxalate is the sole source of carbon and energy.
ODA was determined by redox titration with KMnO4. Titration was performed at different time intervals at two points: the beginning of exponential and the beginning of stationary phases. Thus, we aimed to see at what phase of bacterial growth will be more degraded oxalate – at (Table 3).
Bacteria |
The level of ODA, % |
|||||||
MRS + oxalate (5 g/l) |
Oxalate Medium |
|||||||
Exponential phase |
CI* |
Stationary phase |
CI* |
Exponential phase |
CI* |
Stationary phase |
CI* |
|
Lactobacillus nagelii Z2 |
4 |
0.153 |
10 |
0.835 |
17 |
0.674 |
35 |
0.804 |
Lactobacillus rhamnosus K7 |
2 |
6 |
4 |
7 |
||||
Lactobacillus rhamnosus K8 |
1 |
5 |
3 |
9 |
||||
Lactobacillus frumenti K9 |
5 |
15 |
16 |
23 |
||||
Lactobacillus plantarum S3 |
9 |
13 |
20 |
42 |
||||
Lactobacillus acidophilus S5 |
7 |
11 |
18 |
38 |
||||
Lactobacillus nagelii S12 |
1 |
3 |
2 |
7 |
||||
Table 3 Oxalate-degrading activity of bacteria isolated from different sources determined by KMnO4 redox titration at the beginning of exponential and stationary phases
The most active metabolizer on Oxalate Medium was L. plantarum S3–42% ODA; next in terms of degradation were L. acidophilus S5 and L. nagelii Z2–38% and 35% respectively; the worst results were shown by L. rhamnosus K7 and L. nagelii S12 – both destructed 7% of sodium oxalate.
The Pearson correlation index between percentages of oxalate degradation and OD at λ=540 nm in exponential phase on MRS Broth with sodium oxalate was 0.153, it means that correlation between the 2 variables is weak. In stationary phase at the same medium correlation index was 0.835, so correlation – high. In Oxalate Medium noticed that in stationary phase correlation was higher than in exponential phase – 0.804 and 0.674 responsibly. There is high direct correlation between the growth of bacterial biomass in Oxalate Medium and their ODA. It means that bacteria are more actively degrade oxalate in media where there are no other sources of carbon and energy.
It has been established that from 70 to 80% of stones excreted during urolithiasis are calcium oxalate, and the level of oxaluria depends in a certain way on the composition and functional activity of intestinal microbiota, in particular on the ability to degrade oxalates.12
Except for O. formigenes, a number of normobiote representatives are capable to metabolize oxalate salts (e.g. Lactobacillus spp., Bifidobacterium spp., Eubacterium lentum, Bacillus spp, Enterococcus faecalis). Now scientists are studying the ODA of different strains, as well as developing of probiotics based on microorganisms that are able to metabolize oxalate to prevent urolithiasis.13,14
There are several studies that shown ODA of different Lactobacillus species. But all these studies used different methods for the measuring of the oxalate level and performed their own protocols (sources of oxalate and its concentrations in culture medium, different time of cultivation, samples preparation, etc). There is currently no generally accepted methodology for the determination of ODA. It is widely used high-performance liquid chromatography (HPLC) for the detection of oxalate in samples.
Mogna L et al.,15 detected the efficient ODA of the different Lactobacillus strains. Using HPLC they were investigated that the best oxalate converters is L. gasseri that destruct 68.5% of ammonium oxalate. Next in effectiveness of oxalate destruction were L. acidophilus and L. plantarum–54.2% and 40.3% respectively. In our study we also found the high ODA in two species isolated from Sour cream: L. plantarum S3–42% ODA and L. acidophilus S5–38% ODA.
Campieri et al.,2 identified potential probiotics strains according their ODA. They used pure cultures of L. plantarum, L. acidophilus, L. brevis, Streptococcus thermophilus and Bifidobacterium infantis. The highest percentage of degradation of 10 mM ammonium oxalate showed L. acidophilus–11.8%, and L. brevis showed the lowest–0.9%. S. thermophilus and B. infantis metabolized 2.3% and 5.3% of ammonium oxalate, respectively.
Chamberlain et al.,16 tested the ability of L. acidophilus and L. gasseri to degrade oxalate. Using liquid scintillation counting they are calculate the percent-degradation of the oxalate substrate by these bacteria. The most efficient degrader was L. acidophilus–showed 100% degradation.
Recent studies have shown the efficiency of oxalate-degrading bacteria combined with herbal extracts that reduced the level of urinary oxalate and accumulation of calcium oxalate crystals in the kidney tissue. In the group of rats receiving the plant extract was observed a significant decrease in urinary oxalate levels, but the reduction time in renal inflammation and urinary oxalate was 30 days. Whereas in the group receiving a mix of plant extracts and probiotic bacteria (4 strains of Lactobacillus, 2 strains of Bifidobacterium) was 20 days. This can be a new therapeutic approach to prevent the hyperoxaluria.17
The search for new probiotic strains of bacteria with high ODA is present challenge in the world as well as the development of new methods for estimation of ODA. Our study extends a number of methods for finding new effective bacterial agents to fight against urolithiasis that might have clinical application.
In our studies we adopted redox titration with KMnO4 to evaluate the ODA of bacteria in culture media. This method is highly sensitive and has a low cost. Using this method we determined the ODA of seven Lactobacillus spp isolated from different sources, three of them had a fairly high activity (Lactobacillus plantarum S3–42%, Lactobacillus acidophilus S5–38%, Lactobacillus nagelii Z2–35%).
None.
Authors declare that there is no conflict of interest.

©2020 Akulenko, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.