Journal of
eISSN: 2373-437X


Review Article Volume 7 Issue 4
Department of biological chemistry, Ariel University, Israel
Correspondence: Shimon Shatzmiller, Department of biological chemistry, Ariel University, Ariel, Israel, Tel 0544703583
Received: June 17, 2019 | Published: July 11, 2019
Citation: Shatzmiller S, Zats G, Lapidot I, et al. The microbiome and incurable diseases: discussion review. J Microbiol Exp. 2019;7(4):199-206. DOI: 10.15406/jmen.2019.07.00260
IR, insulin receptor; AD, alzheimer’s disease; IRS, insulin resistance syndrome; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; LPS, Lipopolysaccharide; APP, Amyloid precursor protein; LRP-1, low-density lipoprotein receptor related protein-1; BBB, blood-brain barrier; eNOS, Endothelial nitric oxide synthase; BEC, brain endothelial cell; iNOS, inducible nitric oxide synthase; CNS, central nervous system; nNOS, neuronal nitric oxide synthase; CSF, cerebrospinal fluid; MAPK, mitogen-activated kinase DM1 Diabetes mellitus type I; NVU, neurovascular unit; DM2, Diabetes mellitus type II; OGD, oxygen and glucose deprivation; DM3, Diabetes mellitus type III; PI3, Phosphoinositide-3 kinase; GABA, gamma-aminobutyric acid; GLUT, glucose transporter; PKC, protein kinase C; ICV, Intra cerebro ventricular; TNF, tumor necrosis factor-alpha; IGF, insulin-like growth factor; ILP, insulin-like peptide; LOAD, late-onset sporadic AD
The human microbiota consists of 10-100 trillion symbiotic microbial cells that each person receives, especially intestinal bacteria; Human microbiology consists of the genes found in these cells. Microbial projects around the world have been launched to understand the roles played by this symbiosis and their impact on human health. Just like the question, "What is it to be human?", There are disturbing people from the beginning of recorded history, the question, "What is the human microbiome?" Some researchers have been troubled since the term was coined by Joshua Lederberg in 2001.
The human microbiome, the full array of microorganisms that live on humans and in humans, and more specifically, a collection of microbial genomes that contributes to a broader, or manganese, genetic picture. The genomes that constitute the human microbial represent a wide range of microorganisms that include bacteria, archaea (primitive single-cell organisms), fungi and even some non-living viruses. Bacteria are by far the dominant members of the human microbiology: the bacterial population alone is estimated at 75 trillion to 200 trillion individual organisms, while the human body consists of 50 trillion to 100 trillion somatic cells. The expected microbial abundance indicates that the human body is actually a "gofer-organism," a collection of human and microbial cells and genes, thus combining human and microbiological traits. The scientists demonstrated that “germ-free” mice1-animals that never came into any contact with microorganisms - produced twice the amount of stress hormone (such as cortisol) when distressed than "normal" mice.2 We and practically all living creatures on earth live in symbuios1s with their microbial nemesis, composed of many sorts of microorganisms: bacteria, viruses, fungi, and parasites and many more. Some say that billions of such cells are in us and around us, internally and externally. Now people know that it is not only the gut that has habitat to the microbiome. Also the skin, the blood and even the nervous system. Including brain, inhabit the microbiome. The developing sequencing methods and analytical methods are strengthened our ability to understand the human microbiome, and, indeed, how we define the microbiome and its constituents. In this review we highlight current research that expands our understanding of the microbiome on different spatial and temporal scales. Furthermore, we discuss emerging concepts related to defining operational taxonomic units, diversity indices, core versus transient microbiomes and the possibility of enterotypes. More advances in sequencing technology and in our understanding of the microbiome will provide exciting prospects for exploiting the microbiota for personalized medicine.3
The field of personalized medicine is dominated by genetics, not surprising given our unique DNA, but studies have begun to point to microbial-the community of bacteria, viruses, and microorganisms that inhabit the human body-critical to function and metabolism, and therefore a perfect target. Understand the genetics of microbiome? (Figure 1)
Gut microbiotaA high number of bacteria colonize the human colon. Recent studies have identified about 1000 bacterial species and 7000 bacterial strains for a total of 1013-1014 microorganisms in the gut among the most popular phyla are the Firmicutes,5 Bacteroidetes, which make up 51% and 48% of the whole microbiota. To the phylum Firmicutes, including both Gram-positive and Gram-negative bacteria, belong the genera Lactobacillus (Gram-positive), Eubacterium (Gram-positive) and Clostridium (Gram-positive), although the latter in a minor proportion; on the other hand, the genera Bacteroides and Prevotella belong to the phylum Bacteroidetes, formed by Gram-negative bacteria .The remainder 1% is formed by other phyla, such as Proteobacteria (Gram-negative, in particular the genus Escherichia), Actinobacteria (Gram-positive, particularly the genus Bifidobacterium), Fusobacteria (Gram-negative), Spirochaetes (Gram-negative), Verrucomicrobia (Gram-negative) and Lentispherae (Gram-negative) . To date, it was thought that the microbial gut was only involved in colon-specific activities, such as the fermentation of carbohydrates, vitamin synthesis (e.g. vitamins B and K) and the metabolism of xenobiotics; furthermore, the gut micro flora worked as a functional barrier to prevent pathogenic bacteria from invading the gastrointestinal tract. In recent years, the microbial gut has been greatly re-evaluated in practical terms and mechanisms have been identified in the bi-directional connection with the brain, for which the term gut-brain axis has been coined . Recent studies have shown that microbial alterations can modify normal brain functions resulting in anxiety, depression and cognitive deficits For these reasons, the gut microbiota is considered a real organ, generating a strange paradox as the only organ formed by prokaryotic cells in a eukaryotic organism! The specific contribution of gut microbiota to AD pathogenesis will be discussed in the review hereinafter.
Microbial diversity
It is estimated that human microbiota consists of 900 or 1,000 species of bacteria, making them a diverse collection of microbial genomes. This variety is expressed in changes in the microbial mixtures not only from person to person, but also between compatible body parts, such as the right and left hands of that person. For example, according to one study, a typical hand surface of the hand can contain more than 150 different bacterial species, of which only 17% are shared by the same person and only 13% are shared by different people.
The human intestines are characterized by a high level of microbial diversity and abundance. In a study of 124 European people, researchers isolated about 3.3 million microbial genes. Many of these genes represented species of intestinal bacteria that occur frequently, at least 160 of them thought to live in each person's intestines. The identification of such species that occur frequently in populations is the basis for the definition of standard bacterial cores, which allow scientists to study the interface of human microbial with factors such as diet, culture, and genotype.
The Microbiome at the interface of health and disease
The interest in microbial function in human health has grown over the past decade with the advent of new technologies for the study of diverse microbial communities.6 The large-scale dynamics of the microbiome can be used by many of the tools and observations used in the study of the ecology of the population. The deciphering of the matrix and its accumulated genetic information can also be used to understand the functional features of the microbial community. Microbiome and metagenome must have essential roles in health and disease; their investigation is a boundary in human genetics.
Animals have been living microbes that perform metabolic functions for at least 500 million years, according to a conservative estimate. Extensive cross-fertilization of hosts from animals and microbiota involved in both individual organisms and in total microbial populations, Based on joint adaptation. Interactions between bacteria and their hosts usually involve microbial participation in host functions such as protection, metabolism, and reproduction. For example, comparing mice without conventional mice indicates that microbiota is responsible for most metabolites that are identified in plasma.
Functional variation of the host microbiota can be mediated by the introduction or extinction of some microbial groups or by changing the population structure. These changes can in turn be caused by choice by environmental factors such as dietary changes or exposure to antibiotics. Below we describe the efforts to classify the composition and dynamics of the microbiota. Each human is unique in his/her microbiome. Diseases are accompanied by the alternation of the microbiome blend. If a high-resolution pf the genetic constitution could follow such changes in an accurate way, a novel diagnostic way could be paved in the diagnosis and cure diseases that are uncurable nowadays.
About 50% of the cells in the human body are microbial cells (the human microbiome). Most of these bacteria are part of the microbial of the intestines. Recent studies established a close connection between the composition of bowel microbial and the occurrence of various infectious diseases and complexities, as well as the response to drugs. While many bacteria cannot be identified by known culture-dependent techniques, the next-generation sequence allows the identification of all bacteria in their functional repertoire. Applications of microbiome analysis are diverse and include disease monitoring, microbial biomarker detection or drug development.
A stool sampling kit for a convenient and unbiased self-collection of fecal samples and can be analyzed in our interactive analysis data and further analyzed using user-friendly and freely available software for microbiome data analysis.7
The gut-brain axis (GBA)
The gut-brain axis (GBA) is a two-way connection between the central nervous system (CNS) and the body's nervous system (ENS).8 It includes direct and indirect pathways between cognitive and emotional centers in the brain with peripheral intestinal functions. The GBA includes complex groups between the endocrine (hypothalamic-pituitary) axis, the immune system (cytokines and chemokines) and the autonomic nervous system (ANS).
This axis consists of bi-directional communication between the central nervous system and the gastrointestinal tract, connecting the centers of the brain to the intestinal functions. Recent advances have reported the importance of microbiota in the gut to affect these relations. This connection between microbiota and GBA appears to be directional, that is, by signaling from microbiota to the brain and brain to microbiota through the nervous, endocrine, immune, and humoral systems. Here we summarize the available evidence supporting these interactions, as well as possible pathophysiological mechanisms. Most data were acquired using technical strategies that consist of animal models without bacteria, probiotics, antibiotics, and infection studies. In the clinical clinic, evidence of interactions between microbiota and GBA results from the association of dysbiosis with major neurodegenerative disorders (ie autism, anxiety-depression behaviors) and gastrointestinal (GI) disorders. The irritable bowel syndrome can be considered an example of disruption of these complex relationships, and a better understanding of these changes may provide new targeted therapies.9
Gut microbes start neurodegeneration–The inflammation approach
Neurodegenerative diseases are characterized by selective and progressive degeneration of the neuronal population in the brain, and behavioral, motor, psychiatric, and cognitive associations. The accumulation of pathogenic proteins, mitochondrial function, oxidative stress, transcriptional dysfunction and apoptosis play an essential role in the pathogenesis of degenerative disorders such as Parkinson's disease, Huntington's disease, Alzheimer's disease, and multiple sclerosis. Therefore, innovative treatments directed at each of these mechanisms may be useful in reducing symptoms and slowing down the development and progression of neurodegenerative disorders. This review offers insights into the enormous and diverse benefits of peptides such as neurotrophins, neurotrophic agents (NGF, BDNF, and GDNF), neuropeptides, antioxidants / mitochondrial peptides, MitoQ, and beta splice peptides to treat mechanisms and pathogenesis associated with neurodegenerative disorders.
The association between Parkinson's10 and Irritable Bowel Syndrome (IBS) was caused by a combination of Villanella and Lactobacillus Human microbiologists were investigated.11 It is acknowledged that for gastrointestinal dysfunction function, presence Of clear anatomical or physiological abnormalities should be excluded and that other autonomic dysfunction and neurodegeneration Associated with Parkinson's disease (PD) can be characterized as such abnormalities. Recently there has been recognition An inflammatory component of the pathology of cerebral degeneration, mainly in Alzheimer's disease (AD), but also in Parkinson's patients (PD) and motor neuron disease (MND). The past decades have given opportunities to explore how and inflammation neurodegeneration in the central nervous system are related to each other, although we are still a way to gain a full understanding of the complex interactions that can occur.12 Gut microbiome13 is involved in obesity, for example.14 There develop different attitudes for the treatments and cure of neurodegenerative diseases like Parkinson's and Alzheimer's disease.15
Antimicrobial peptides and their surrogates might be a way to combat neurodegenerative diseases.16 Neurodegenerative diseases such as type 2 diabetes, obesity; Parkinson's disease (PD) and Alzheimer's disease (AD) represent the most significant challenges to global health this century, and are associated with significant comorbid morbidity and health costs. Although many factors undoubtedly contribute to the development and development of obesity and obesity, research over the past decade has shown that the bacteria surrounding the human intestines may play critical roles. Gout bacteria are now known to be partners with the human host, and are primarily affected by the state of birth and early nutrition and nutrition, as well as environmental and other factors, including exposure to antibiotics. Intestinal bacteria contribute to human health through roles of diabetes breakdown, nutrient uptake, inflammatory reactions, intestinal permeability, and alteration of bile acid. Many studies have shown that disruptions in the relative proportions of gut intestines may contribute to weight gain and insulin resistance, including changes in Gammaproteobacteria and Verrucomicrobia and changes between Bramoidets and Bacteroidetes in weight gain and possible changes in butyrate-producing bacteria such as palliative bacteria, prausnitzii in DM. It has also been shown that methanogenic archaea may contribute to metabolism change and host weight gain. However, most studies are performed with stool or colon samples and cannot be representative of the small active metabolic intestine. Studies mainly in rodent models begin to elucidate the mechanisms by which intestinal bacteria contribute to DM and obesity, but much remains to be learned before we can begin to approach targeted therapies.
Currently, AMPs represent one of the most promising future strategies for combating antimicrobial drug resistance infections due to their bacterial and microbial mechanism by wall cell disruption. This is evident by the growing number of studies to which these peptides have been studied. It is clear: our need for new antimicrobial becomes more urgent due to nosocomial infections and inadequacy is currently applied to the agent to fight resistant bacteria on the one hand. On the other hand neurodegenerative diseases are believed to begin with inflammation and infection which may require treatment with modified AMPs. The question arises: Can we develop new drugs based on the principles of the design of primitive molecules? The advantage of AMP is that most of them operate in a broad spectrum of bacteria in a new mechanism that is less likely to allow the development of resistant strands of bacteria. However, at present many mechanisms[4], have many drawbacks. Difficulties identified when AMPs. These substances are considered as potential treatment agents. Drug-like compounds based on antimicrobial drugs (AMP) suffer from one central deficiency that can jeopardize efforts for therapeutic use: they are not "selectively strained",17 the microbial "good" and the "bad" bacteria are similarly killed.18\ The elimination of all bacteria occurring is of similar efficacy.19,20 To overcome the disadvantages and present features that do not exist in the AMPs themselves. Some of the famous AMPs are isolated from the skin of African Todd, Magazines. The company established with the same name understood the therapeutic potential of antimicrobial imitations of the natural magazine, namely polypeptide of semi synthetic pexiganan21 as analogous to the magazine. Agent’s emulsions that are imitations of AMPs are called collective peptidomimetics. The types of changes that are presented are usually based on the structural requirements that are known to affect AMP activity. Attempts to preserve favorable charge properties like amphipathicity are performed to ensure the antibacterial activity of mimicking compounds. The mimics, often constructed with another spine (ie, not based on amino acids), may or may carry disconnected side chains to overcome the low bioavailability and metabolic instability found for traditional AMPs.22
Genome-wide association study (GWA study, or GWAS)
The microbial involvement23 in health and disease is well established. Microbiome genome-wide association studies (GWAS) are used to elucidate the interaction of host genetic variation with a microbiome. The emergence of this relatively new field was triggered by the advent of next-generation sequencing technologies that enable investigation of the complex interaction between host genetics and bacterial communities. In this paper, we are investigating recent studies of interactions between the host microbial using mGWAS. In addition, we emphasize the gap marked in the sample population of mGWAS currently conducted and draw attention to the critical need for including diverse populations. The involvement of microbial in health and disease, and the complexity of its composition and its function, is impressive in its reference to human genetic factors that influence the microbial composition.24 Genes may affect health by their ability to promote a stable microbial community in the gut. Permission studies yield a consistent subset of germs affected by genes, but the use of genome association studies (GWAS) to identify specific genetic variants associated with macrobiotics phenotypes has shown a challenge. Processing microbiome datasets into features to be modeled and reducing the burden of multiple tests are just some of the technical obstacles of GWAS microbiome. Studies to date are small by GWAS standards, making cross-validation comparisons and validations particularly crucial in identifying authentic signals. Cross-disciplinary comparisons make it difficult to distinguish between analytic approaches. However, some consistent associations appeared between the populations, mainly between bifidobacteria and the lactase non-persister genotype. These early successes open the way for microbial integration into studies that involve genotype, environment, and microbial interactions to predict susceptibility to disease.
Genome-wide association studies of the human gut microbiota, use of GWAS
The microbial composition of human fecal feces is affected by many lifestyle Factors, especially diet.25 It is less obvious, however, what role the host genetics play in dictating the Composition of bacteria living in the intestines. Researchers examined the association of ~ 200K host genotypes with the relative abundance of bacterial and fecal doses in the founder population, Hutterites, during two seasons (n=91 summer, n=93 winter, n=57 people Collected in both). These people live and eat communally, minimizing variance Environmental exposures, including diet, that may disguise small genetic phenomena. Using the GWAS approach which takes into account the relationship between the subjects, scientists Identified at least 8 bacterial species whose abundance was associated with single nucleotide polymorphisms in the host genome in each season (in a wide-FDR genome of 20%).
Although the research results indicate that host genetics plays a role in determining the composition of the intestinal microbiome, there are some limitations. The first is the relatively small size of our sample (n 100 people per season). For many GWAS of common diseases, the sample sizes needed to identify significant associations are thousands to tens of thousands of people. We suggested that the relatively stable environment in which the species are exposed, especially their community diets, will reduce variability caused by non-genetic factors and increase our power to detect genetic associations. It is therefore clear that much larger sample size will be needed to ensure sufficient strength to identify the relationship with multiple high testing loads and to reduce safety margins on parental estimates. A second limitation is that replicated copies are not currently available for these features in humans, and we cannot confirm any associations we identify in independent human samples. Despite these limitations, we do replicate previously observed QTL bacteria observed in the mouse. In addition, analysis of candidate tissue identification provided additional validation of our results, since we would not expect any kind of enrichment if our results were forged. Finally, several lines of evidence indicate a relationship of type of acramasonia, in particular, to host genetics, including sizeable GWAS-wide genome hits, endothelial cell enrichment for top associations. Given the medical importance of this type, further work needs to be done to confirm this association and to investigate further how host genetics may influence this conclusion. This study is one of the first to investigate host genetic effects on the abdominal microbiome composition on a wide-scale genome in humans. We have identified bacterial species that exhibit specific patterns of sex abound in Hutterites (An imaginary disease. The Catholic Church, The Lutheran Church and Reformed Churches of John Calvin and Ulrich Zwingli frequently charged the Hutterites with heresy), although it is unclear whether biological or cultural factors lead to these patterns. We have identified at least 10 bacterial taxis in each season that appear to be effective by examining "chip indications". At least seven bacterial traits per season are related to human genome change at a genomic significance level when examining the number of SNPs tested within each GWAS. Genetic enrichment analysis showed that SNPs identified in these GWAS are likely to function through a variety of different body mechanisms including immune function, metabolism, and energy regulation. Finally, the candidate tissues where the host genetic variation can act to influence the microbial abundance in the gut have been identified. This work offers the first attempt into the role where human genetics plays in maintaining the involvement of the microbiome.
Pharmacogenetics and the concept of individualized medicine
The perception that every individual human life in symbiosis with a characteristic microbiome, and that the blend and constitution of these billions of microbes are sensitive to the health condition of the individual, suggests that this could be applied for the new concept of individualized medicine.26
A negative response to the drug in patients causes more than 2 million hospitalizations including 100 000 deaths yearly in the United States. This is a contrary remedy the response can be due to multiple factors such as disease determinants, Environmental and genetic factors. To improve efficiency Safety and understand the tendency and clinical outcomes of drugs, two fields in rapid development - pharmacogenetics (the focus is on individual genes) And Pharmacogenomics (the focus is on many genes)-have done studies on the genetic customization of drug response. It's because of many drugs It seems that the responses were genetically determined and the relationship between them Genotype and drug reaction may be valuable diagnostic and very expensive. Identification and characterization of a large number of genetic polymorphisms (biomarkers) in drug and drug-enzyme enzymes a diverse ethnic group of people may provide necessary knowledge On the mechanisms of interpersonal differences in drug response. However, progress in understanding complex diseases, its negative psychosocial consequences, violation of privacy or discrimination, cost associated Availability and complexity (extensive geographical changes in genes) May become possible barriers in the integration of these pharmacokinetic data Risk assessment and treatment decisions. In addition, it requires increased enthusiasm and education in the clinical community and understanding of pharmacognosy itself by the lay public. Although personal remedies remain a challenge for the future, the pharmacokinetic approach to drug development should be continued. If it becomes in reality, it provides benefits to improve public health and allow genetically sub-group diseases and thus avoid adverse side effects (by knowing in advance who should be treated with some medication and how).
Individualized medicine and the microbiome in the reproductive tract
Humans have evolved along with millions of microorganisms that inhabit their bodies.27 These bacteria outperform human cells by 10 to 1 and tell about 3×106 genes, more than ten times the 25,000 human genes. This microbial antigen acts as our "other genome" and, like our genes, is unique to humans. Recent international efforts, such as the Microbiome Human (HMP) project and the Meta HIT project, have helped to classify this bacterial genome by sequencing the next generation of culture. This describes recent efforts to define a variety of bacteria in the female reproductive system because of the effect that microbial function has on reproductive efficiency. In this review we will discuss the current evidence that microbial communities are critical to maintaining fertility health and how disorders of microbial community structures can affect reproductive health from the aspect of infection, reproductive cycle, pregnancy and disease. Investigations of the human microbial drive intervention strategies in treating medical populations for the treatment of isolated patients. In particular, we emphasize how understanding and defining microbial structures in different diseases and physiological situations lead to the discovery of biomarkers and, more importantly, the development and implementation of microbiological intervention strategies for modern medicine. Finally, this review will conclude with a summary of the literature on the effectiveness of microbial intervention strategies applied in animal and human models of the disease and the potential for integrating these microbial intervention strategies into standard clinical practice.
This approach is based on the literature that has expanded so far that the microbiology of the individual serves more than just a bacterial presence that resides in the natural bodies of our body. These microbial environments consist of many communities that act to create symbiotic-host-host relationships by regulating the immune system and normal physiology of the disease. Microbial community dynamics may vary in diversity and wealth, but can maintain a standard "microbial" community due to redundancy among specific species. However, too many vibrations in each microbial community leave the host susceptible not only to overacting bacterial colonization, but also to enter the pathogen. In this case, there can be severe damage to the cellular layers and tissue of a physiological system that can lead to multiple systemic system inhibitors, including reproductive efficiency.
In this article we have highlighted literature indicating that communication between the host immune system and the bacterial communities residing in the vaginal cavity affect reproductive health. Further clarification of host bacterial activity within the vaginal cavity will increase our understanding of the pathogenesis of reproductive diseases and possible causes of fertility impairment. In general, the continuous and continuous screening of human microbial will provide early biomarkers in patients who are "normal" before clinical symptoms. It is more important to understand what is and how to achieve the "perfect" balance of human microbiomes and will provide the best medical weapon for individualized personalized medicine for the prophylactic treatment of many pathogenic diseases.
Treating neurodegeneration in the microbiome way
The penetration of gut microbiome flora via the leaky gut and damaged Blood-Brain-Barrier can cause inner-brain inflammation and subsequent infection.28 The organism defends itself against this microbial invasion by the degradation of some proteins and polypeptides like insulin, amylin or amyloid precursor peptide (APP), synuclein, for example, and other digestive peptides to antimicrobial peptides, like beta-amyloid, to slow or halt the progress of the microbial inflammation and infections causing the brain damage, namely the killing of the brain cells in a slow process characteristic to neurodegenerative diseases. The amyloids have also a negative face: they aggregate to plaques tangles and other polymeric insoluble deposits which damage the neurons and interfere in the transmission of the nerve signals at the synapses. It this is the case, other short peptides or their surrogates may combat the microbiome but will be designed to structures that will prevent aggregation in the brain. In such circumstances, peptide surrogates might become instrumental.
How the microbiome affects Alzheimer's disease
One important scientific discoveries in recent years have been the discovery that the intestinal microflora takes part in two-way communication between the intestines and the brain.29 Scientists show that human intestinal microflora may even act as a the second brain should be responsible for neurodegenerative disorders such as Alzheimer's disease (AD). Despite the human-related Bacteria communities are generally stable, they can be modified by everyday human actions and experiences. Antibacterial bacteria, Complement, pathogenic microorganisms, may have a significant impact on the immune system, brain development, and behavior, such as They are capable of producing several neurotransmitters and neuro-modulators like serotonin, kynurenine, catecholamine, etc., as well as Amyloids. However, brain destruction mechanisms, which can lead to AD dementia, start with the intestinal microbiome dysbiosis, development of local and systemic inflammation, and dysregulation of the intestinal brain axis. Increased permeability of the colon epithelial barrier results neuro-inflammatory reactions in the brain. It seems that, the infectious hypothesis of AD, with a considerable role of Gut microbial, begins to gently push into the hypothetical shadow of the amyloid cascade that has dominated for decades. It strongly assumes that AD can start in the intestines, and is closely related to the imbalance of microbiota in the intestines. This is a promising area for therapeutic intervention. Modulation of microbiota in the intestines using a personalized diet or microbiota intervention is beneficial, change Microbial partners and their products, including amyloid protein, will probably become a new treatment for AD (Figures 2 & 3).
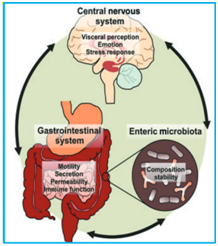
Figure 3 Representation of the enteric microbiota-gut-brain axis and how anxiety-like behavior can be measured using the elevated plus maze. Recent studies have shown that perturbations of the enteric microbiota can impact on this axis to alter behavioral responses in animal models.
Human microbiology consists of symbiotic microorganisms that constitute a highly complex and dynamic ecosystem:30 the GI is the largest reservoir of human microbiology to date, and its impact on human health and neurological diseases is growing. Bacteroidetes, the most abundant bacterium of Gram-negative bacteria in the gastrointestinal microbiome, while ordinarily beneficial to the host when confined to the gastrointestinal tract, has the potential to secrete an incredibly complex array of pro-inflammatory neurotoxins including surface lipysyscharides (LPSs) and toxic proteolytic peptides. The harmful effects of these bacteria exudates become more critical as GI brain ways and blood barriers change or increase their permeability with aging and disease. For example, the presence of unique LPSs of Bacteroidetes in abundance and Fragilis Bacteroides (BF-LPS) serum represents a significant cause of systemic inflammation. BF-LPS is further recognized by receptors of TLR2 cells, TLR4 and/or CD14 in microglial cells, as well as 42 peptides of amyloid-beta amino acids (AB42) and brain characteristics of Alzheimer's (AD). Here we provide the evidence that exposure to BF-LPS cells in the primary human brain is a particularly potent inducer factor of pro-inflammatory transcription factor NF-kB (p50/p65) complex, a known cause of expression of pathogenic pathways involved in inflammatory cerebral degeneration. This "communication perspectives" will also highlight work from recent studies that promote novel evolving concepts on the potential contribution of microbiome-generated factors, such as BF-LPS, in pro-inflammatory neurodevelopmental brain neurodegenerative AD.
Diabetes mellitus DM2
Studies have shown that diabetes predicts independent dementia of white substance hyperintensities (WHI). In addition, the assumption that WHI is proxies of ischemic brain disease is unclear, since WHI can represent neurodegeneration and cerebral amyloid [59]. Our research strength includes the longitudinal nature, and the detailed characterization of late Alzheimer's disease (LOAD).It was previously observed in the years 1992-1994 that the history of T2D was closely associated with a higher risk of dementia, including LOAD.
Type 2 diabetes has been a reason to increase the risk of both vascular dementia and Alzheimer's disease (AD). Several studies have suggested that AD is "type 3 diabetes".31,32 Evidence for the association between diabetes and neuropathology of AD is less clear. Little is known about the mechanistic association between type 2 diabetes mellitus (T2DM) and Alzheimer's disease (AD).33 The causes of complications caused by diabetes are CNS multifactorial and they understand relatively little although it is now clear, that the blood-brain barrier (BBB) damage plays a significant role in CNS-dependent diabetes disorders. Changes in glucose levels in the plasma (hyper or Hypoglycemia) were associated with BBB transport functions changed (Such as glucose, insulin, choline, amino acids, etc.), integrity (tight junction) Interference), and oxidative stress of CNS microcapillaries. The last two Indicating potential causal potentials for regulation and activity Receptor for advanced end glycation products (RAGE). That's me The protein membrane also transports amyloid beta (Aβ) from the blood Into the brain over the BBB thus, creating a connection between type 2 Diabetes (T2DM) and Alzheimer's disease (AD, also called Such as "type 3 diabetes"). Hyperglycemia is associated with progression of cerebral ischemia and secondary improvement of the secondary brain injury.
Neurodegenerative Parkinson’s disease may be viewed in microbiome spectacles as a result of gut inflammation
A model of PD pathogenesis, originating from colitis. In a sensitive person, inflammatory factors34 initiate immune responses in the harmful intestines of microbiota, increase intestinal permeability, and increase expression and accumulation of αSYN (2). Sinolinopathy may be transmitted from the intestines to the brain through the vaginal nerve (3b), and intestinal inflammation and chronic infiltrations promote systemic inflammation, which, among other things, can increase the permeability of the blood barrier in the blood (3a). Intestinal inflammation, systemic inflammation, and pathology of cerebral syncholines promote neurodynamics (4), which inhibits the neurodevelopment of PD (5).
Scientists at the University of Alabama35 found similar results in 197 patients with PD and 130 healthy controls. Patients with PD have evidence of a microbial of unbalanced intestines in which several species of bacteria exist in more significant numbers and some in smaller numbers than healthy individuals. These studies are just a few of the many investigate the relationship between intestinal bacteria and Parkinson's disease. Further investigations are also ongoing on possible connections between intestinal microbial and other neurological diseases, such as dementia and autism.
Human micro bites include 10-100 trillion symbiotic microbial cells. These bacteria (bacteria, fungi and parasites) live on the human body and their number exceeds our cells 10:1. The delicate balance of human microbial is essential for good health. Adversely affect the immune system, vaginal and respiratory health and especially, stomach. It is estimated that the gastrointestinal tract stores more than 500 species of bacteria, and that the bacterial genome exceeds the human genome in the factor of 100:1.36 Interference of the gut microbial was involved in diarrhea, functional constipation, irritable bowel syndrome and inflammatory conditions of the system Such as inflammatory bowel disease (IBD), Crohn's disease and ulcerative colitis, and neurodegenerative diseases Alzheimer's, Parkinson's, Diabetes 2 for example.
None.
Authors declare that there is no conflict of interest.

©2019 Shatzmiller, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.