Journal of
eISSN: 2373-437X


Research Article Volume 11 Issue 5
Belarusian research center for pediatric oncology, hematology, and immunology, Republic of Belarus
Correspondence: Sviatlana L Kandaurava, Belarusian research center for pediatric oncology, hematology, and immunology, Minsk, Republic of Belarus
Received: December 12, 2023 | Published: December 28, 2023
Citation: Niafiodava A, Tchernovetski M, Kаndaurava S, et al. The etiological structure of mucormycetes isolated from children with oncohematological pathology with description of the clinical case of invasive mucomycosis in patients with impaired immunity. J Microbiol Exp. 2023;11(5):143-148. DOI: 10.15406/jmen.2023.11.00402
Introduction: Mucormycosis is one of the fungal infections caused by fungi (mucormycetes) belonging to the order Mucorales and are mainly part of the genera Apophysomyces, Cuniiinghamella, Lichthemia [formerly Absidia], Mucor, Rhizopus, Rhizomucor, Saksenaea. The causative agents of mucormycosis are widespread in the environment. Fungal infection is manifested by rapidly developing lesions of the skin, mucous membranes, internal organs, and tissues. The most common forms of infection are the rhinoorbital-cerebral and pulmonary forms caused by pathogens of mucormycosis. Gastrointestinal, skin, and disseminated forms of fungal diseases are also registered. The mortality rate in mucormycosis reaches 70% in some cases and depends on the clinical form and the timeliness of the therapy initiated. The diagnosis of mucoromycosis is based on the complex application of various approaches including traditional seeding of biological material, histological analysis of material from affected loci, X-ray, and molecular biological studies. In terms of the treatment of this type of fungal infection, the use of amphotericin B, posaconazole, izavuconazole in combination with surgical rehabilitation of affected tissues has the greatest effectiveness.
Purpose: Identification and analysis of the etiological structure of mucormycetes isolated from children with oncohematological pathology and impaired immunity as well as a description of the clinical case of concomitant generalized gastrointestinal mucormycosis against the background of the underlying disease associated with disorder of the immune system.
Material and methods: The study includes the results of the isolation of mold fungi (including pathogens of mucoromycosis) from clinically significant biological material for the period from January 2002 to June 2023. To isolate the fungal microflora, traditional microbiological sowing of biomaterial was used followed by generic and specific (in some cases) identification using phenotypic, molecular biological, and mass-spectrometric types of laboratory analysis. When describing the case of invasive mucormycosis, methods of endoscopic and pathomorphological diagnostics were additionally used.
Results: The etiological structure of mucoromycetes identified in children with oncohematological pathology and impaired immunity has been studied. As a result, 85 strains of mycelial microflora were isolated from clinically significant biological material obtained from the respiratory organs and respiratory tract (lung biopsies, contents of bronchoalveolar lavage (BAL), and tracheobronchial drainage), which accounted for 30.69% of 277 identified mold micromycetes. At the same time, 12 strains of mucormycetes were identified, which accounted for 14.12% of the total number of fungi found. The isolated mucormycetes were representatives of the genera Mucor, Rhizopus, Rhizomucor, and Lichtheimia [Absidia]. There were also the isolated cases of detection of mucoromycosis pathogens in blood (one strain of Mucor spp. and one strain of Rhizopus spp. out of 222 hemocultures), in urine (one strain of Mucor out of 113 ureacultures), in liver biopsies (one strain of Rhizopus spp.) and gastrointestinal (two strains of Rhizopus microsporus). The clinical case of invasive mucormycosis of the gastrointestinal tract (GI tract), which occurred in a patient with Nijmegen syndrome in the post-transplant period against the background of a coronavirus infection, is described.
Conclusion: The total proportion of mucormycosis pathogens (detected in the lungs and in the contents of the respiratory tract) among the total number of filamentous micromycetes reaches 14.12%. The complex application of various diagnostic approaches (including microbiological seeding of biomaterial, X-ray, pathohistochemical and molecular biological studies) makes it possible to reliably verify invasive mucormycosis. In patients with immune defects, despite intensive antifungal therapy, mucormycetes can cause severe forms of invasive fungal infection, up to a fatal outcome.
Keywords: mucormycosis, micromycetes, impaired immunity, children
Among the wide variety of fungal mycelial pathogens that cause infectious of organs and tissues in humans, representatives of the order Mucorales occupy a special place. The main factors contributing to the emergence of various forms of mucormycosis are: impaired immunity against the background of the underlying disease in the form of oncohematological pathology and congenital defects of the immune system; long-term use of antibiotics and corticosteroid drugs; organic lesions of the lung tissue; diabetes; coronavirus infection.1−4 At the same time, invasive lesions of internal organs and tissues in mucormycosis are among the most severe and life threatening to the patients. The mortality rate in this form of pathology can be of 30-70% and higher.5,6 For the first time, a human disease with mucormycosis was described as "mucorinic mycosis" by Professor Paltauf in 1885. Based on his drawings of the identified etiological agent, with sporangiophores and rhizoidal structures, it is suggested that the infection was most likely caused by Lichtheimia corymbifera.7 Today, the total number of identified fungi of the order Mucorales is over 260 species belonging to 55 genera.8 The main pathogens for humans are representatives of the genus Rhizopus, in particular, Rhizopus arhizzus (R. oryzae) and Rhizopus microspores.9 The causative agents of mucormycosis are rarely detected in the blood, in most cases, they are detected in the contents of the respiratory tract and respiratory organs. Therefore, to isolate this type of mold microflora, sputum, bronchial washings, contents of bronchoalveolar lavage (BAL) and tracheobronchial drainage, biopsy specimens of the lug are most often used.10,11 Taking into account the possible contamination of biological material during its collection, the most informative are the microbiological seedings from the samples of the biopsy specimens of the lung, as well as from the contents of BAL and tracheobronchial drainage.12
Taking into consideration the clinical severity of mucormycosis and the high probability of an unfavorable outcome of the disease, it is of fundamental importance to carry out a set of measures for the early diagnosis of mycosis and the appointment of the adequate antifungal therapy. For this purpose first of all, classical microbiological studies aimed at isolation, phenotypic identification, and determination of antimycotic sensitivity of the etiological agent of the disease are used. The mass spectrometric analysis of the protein components of micromycetes using the MALDI-TOF technology (matrix-activated laser desorption/ionization with a time delay) has been well established. If it is possible to take the biopsy specimens of the lung and the material from other clinically significant loci, a histochemical analysis of tissue samples is performed. Along with the laboratory tests, the X-ray diagnostics of lung lesions is carried out using X-ray and computed tomography, methods of endoscopic and pathomorphological diagnostics.13,14
The introduction of molecular biological technologies into practical healthcare aimed at the qualitative and quantitative detection of the specific regions of the genomes of fungal strains in biomaterial samples has high expectations.15−17
However, the limited therapeutic options for the treatment of mucormycosis are of particular concern. Currently for this purpose, certain drugs of polyene series and azole group are effectively used.18,19 Also surgical debridement of affected tissues is used.
The aim of the study was to determine the proportion and analyze the etiological structure of mucormycetes isolated from the children’s respiratory system with concomitant infectious complications against the background of the main oncohematological pathology or pathology of the immune system as well as to describe the clinical case of invasive mucormycosis of the GI tract, diagnosed with the integrated clinical and laboratory approach.
To isolate mold micromycetes, biological materials obtained by draining the tracheobronchial tree (TBD), conducting a bronchoalveolar lavage (BAL), and lung biopsy specimens were used. When diagnosing the described case of gastrointestinal mucormycosis, the biological samples from the affected areas of the GI tract were also examined. Biomaterial samples were collected under aseptic conditions into sterile containers, immediately delivered (no later than 2 hours) to the laboratory, and seeded on solid nutrient media in the Petri dishes. Then thermal incubation of the studied samples with daily visual control of fungal cultures growth was carried out. Sabouraud Dextrose Agar (with gentamicin and laevomycetin) and Czapek-Dox Modified Agar were used as the main nutrient media. Along with inoculation, primary microscopy of native biomaterial smears was performed using the standard Gram stain and/or methylene blue stain as well as additional stains such as Calcofluor White. Depending on the stain used, light, phase-contrast or luminescence microscopy of the smear was performed at 100x magnification of the test sample. Incubation of cultures on Sabouraud Dextrose Agar and Czapek-Dox Modified Agar was conducted under aerobic thermostating at a temperature of +30±2°C for at least 14 days. The main purpose of inoculation of biological material in the Petri dishes with selective nutrient media was to grow isolated colonies of the so-called "pure" culture of the potential pathogens of mycoses. The subsequent primary identification of the grown mycelial microflora was carried out phenotypically by assessing: the nature of the growth of microorganisms; type, consistency, texture, and color of fungal colonies; temperature regime and the length of thermal incubation of micromycetes; presence of filamentous structures in the smear. In order to determine or confirm the generic and species affiliation of grown cultures of mold fungi, repeated microscopy of smears of isolated colonies was conducted (with an assessment of the configuration and septation of the mycelium; presence of rhizoids; the shape of vegetative structures in the form of sporangiophores with sporangia and sporangiospores), mass spectrometry of grown strains of mold micromycetes using MALDI-TOF MS technology in an automatic spectrophotometer Vitek MS (BioMerieux, France) was also conducted.
All histological specimens were examined by light microscopy. Raw material was fixed in a 10% neutral formalin solution. After this, tissue sections were cut into pieces 0.3 cm thick and placed in slides. Histological processing was conductes in an automatic tissue processor Microm STP420. Then the tissue pieces were placed in paraffin blocks, cut with a microtome, and the serial sections were stained with hematoxylin-eosin. The material of the most representative area was additionally stained according to the Schiff test.
The sensitivity of filamentous fungi to antifungal drugs was assessed by ellipsometry (E-test type). The results of minimum inhibitory concentrations (MICs) were evaluated using the European Committee for Antimicrobial Susceptibility Testing (EUCAST) expert rules.
The analysis of the results of microbiological inoculation of samples of BAL and TBD contents, lung biopsies contents for the period from January 2002 to June 2023 was carried out in order to assess the etiological structure of the isolated mold microflora. In total, 277 strains of micromycetes were isolated, 85 strains of which belonged to filamentous fungi (30.69%) (Table 1).
№ |
Name of microorganism |
Amount |
Percentage% |
1 |
Aspergillus spp. |
59 |
69.41 |
2 |
Mucor spp. |
6 |
7.05 |
3 |
Rhizopus spp. (including 2 strain R. microspores and 1 strain R. arrhizus) |
4 |
4.71 |
4 |
Rhizomucor pusillus. |
1 |
1.18 |
5 |
Lichtheimia (formerly Absidia) corymbifera |
1 |
1.18 |
6 |
Penicillium spp. |
5 |
5.88 |
7 |
Trihophyton spp. |
1 |
1.18 |
8 |
Paecilomyces variottii |
2 |
2.35 |
9 |
Purpureoccillum liliacinum |
1 |
1.18 |
10 |
Scedosporium spp. (including 1 strain Sc. apiospermum) |
2 |
2.35 |
11 |
Undifferentiated mold |
3 |
3.53 |
TOTAL: |
|
85 |
100.0 |
Table 1 Detection of mold in the contents of the respiratory tract and respiratory organs in children with oncohematological pathology for the period from January 2002 to June 2023
Representatives of the genus Aspergillus prevailed and were detected in 59 (69.41%) out of the 85 identified strains of mold. The total number of mucormycosis pathogens isolated from the lungs and the contents of the respiratory tract in the form of Mucor spp., Rhizopus spp. (including 2 strain R. microsporus and 1 strain R. arrhizus), Rhizomucor pusillus and Lichtheimia [Absidia] corymbifera is 12 strains (14.12%) out of 85 mold cultures. The obtained data indicate the urgency of the problem of pulmonary forms of mucormycosis in children with the underlying oncohematological pathology.
Patient T., 9 years old, diagnosis: congenital defect of immunity was first diagnosed in 2019-Nijmegen breakage syndrome (a mutation in the NBS gene was verified in June, 2019). Hypogammaglobulinemia.
Nijmegen Breakage Syndrome (NBS) is an autosomal recessive disorder that is a combination of immunodeficiency, hypersensitivity to X-rays, high predisposition to oncopathology and autoimmune diseases, and it is characterized by phenotypic features (microcephaly from birth and/or intrauterine, "bird-like" features, hypo- and hyperpigmented spots on the skin). One of the main clinical manifestations of NBS (in addition to microcephaly and skin manifestations) is recurrent infections from an early age: frequent viral respiratory infections, less often otitis, enterocolitis, urinary tract infections, stomatitis, chronic bronchitis that are caused by the defects in humoral and cellular immunity. Moderate leukopenia and lymphopenia are detected. The number of CD3+, CD19+, CD4+ cells, CD4+/CD8+ ratio, the levels of immunoglobulins (Ig) A and G are decreased, which can contribute to the protracted course of concomitant viral infections (including COVID-19). Taking into account the variability of the phenotype in NBS, even in patients with non-severe immunodeficiency, the course of the disease must be predicted with great caution. In NBS the recurrent infections, which against the background of immunodeficiency usually quickly pass into generalized forms, are of particular concern. Fatality can occur from septic complications. Due to prolonged infections, some children develop amyloidosis, which causes chronic kidney failure and can leads to death. The only effective treatment is hematopoietic stem cell transplantation.
Due to the underlying disease, Patient T was prescribed regular replacement therapy with intravenous immunoglobulin at a dose of 0.4 g/kg 1 time every 3–4 weeks. It was decided to search for an unrelated donor in the Republic of Belarus and abroad with the aim of further transplantation of hematopoietic stem cells. During the monitoring period, the patient systematically received intravenous immunoglobulin, antimicrobial and antiviral therapy (due to the presence of chronic recurrent EBV infection).
At the time of transplantation, the patient had chronic recurrent EBV infection (PCR of peripheral blood DNA EBV-7075 copies), infiltrative changes in the middle lobe of the right lung, and thickening of the mucosa of both maxillary sinuses according to CT scan.
After the patient was prepared and examined in December 2021, he underwent allogeneic unrelated HLA-compatible bone marrow hematopoietic stem-cell transplantation (HSCT).
The first signs of deterioration appeared on day +14 after transplantation. The patient had the following symptoms: febrile temperature, mucositis, gingivitis, dermatitis, and colitis. Antibacterial therapy was prescribed (cefepime with further replacement with meropenem, teicoplanin in the age dosage). A blood test for galactomannan, a structural component of the cell wall of mold, was positive (optical density index-1.94).
Microbiological cultures did not give the growth of mold fungi. Taking into consideration the severity of the patient’s condition, engraftment syndrome as well as signs of infiltrative changes in the middle lobe of the right lung, and a positive serological blood test for galactomannan, a diagnosis was made: probable pulmonary aspergillosis (on day +14 after transplantation). Voriconazole 200 mg 2 times a day intravenously (8 mg/kg/day 2 time per day) was prescribed. During the control study, the residual serum trough concentration of voriconazole was 2.3 µg/ml (the target therapeutic concentration according to international recommendations is 1–5.5 µg/ml).20 Thereafter, the determination of galactomannan in the blood serum was carried out systematically with a frequency of 1 time per week. In order to prevent graft-versus-host disease (GVHD), starting from the first day of hematopoietic stem-cell transplantation (HSCT), the patient received cyclosporine and mycophenolate mofetil.
2.5 months after HSCT, the patient was diagnosed with COVID-19 infection (+45 days after HSCT). At the time of infection, the patient received immunosuppressive (cyclosporine), antibacterial (meronem 120 mg/kg/day, vancomycin 60 mg/kg/day), antifungal (voriconazole 8 mg/kg/day), and full accompanying therapy. The patient received remdesivir (5 mg/kg 1 day, 2-5 days 2.5 mg/kg, total 396 mg) according to the order of the Ministry of Health of the Republic of Belarus dated 24.06.2022 No. 858. On the +60th day after HSCT, CT scan of the chest showed negative changes in pneumatization of both maxillary sinuses, pansinusitis. Visible pathological changes in the chest organs were not detected.
On the +100th day after transplantation, there were still negative changes in pneumatization of both maxillary sinuses-pansinusitis, and there were areas of lung tissue consolidation in S3 on the right. The patient still had signs of COVID-19 infection (positive PCR result). The main complaints were febrile fever and loose stools up to 10 times a day. CT scan showed bilateral interstitial-infiltrative changes in the lungs, bilateral hydrothorax (worse on the right side), lymphadenopathy of all groups of mediastinal and axillary lymph nodes with induration of the mediastinal tissue.
Voriconazole was replaced with caspofungin intravenously (45 mg per day, equivalent to 50 mg/m2/day). Follow-up determination of galactomannan in the blood serum revealed a negative value of the optical density index (0.47). However, against the background of ongoing therapy, the patient's condition continued to worsen mainly due to the irritable bowel syndrome (very frequent stools up to 2-3 l/day, loose, watery, sometimes with mucus). Red staining of the stool was reported, which raised the suspicion of gastrointestinal bleeding. In this regard, it was decided to perform esophagogastroduodenoscopy (EGD) and colonoscopy. According to the results, the endoscopic picture revealed the manifestations of the intestinal form of the graft-versus-host disease (GVHD) (Figure 1, 2). The patient was prescribed methylprednisolone at a dosage of 1 mg/kg/day followed by an increase in the dose to 2 mg/kg/day.
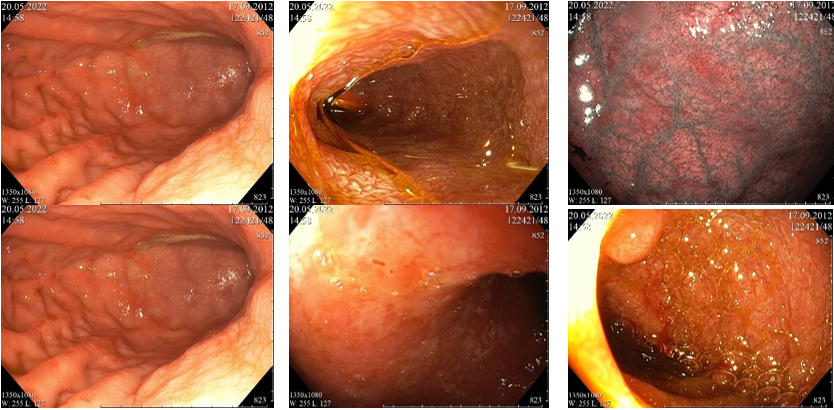
Figure 1 Esophagogastroduodenoscopy images. Moderate erythematous pangastropathy with punctate rash, erosive bulbitis, erythematous duodenopathy with moderate lymphostasis. The endoscopic picture may correspond to the manifestations of the intestinal form of GVHD.
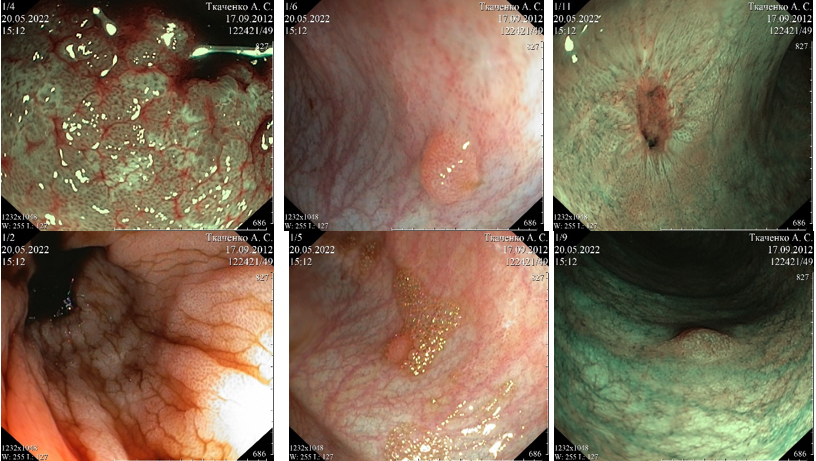
Figure 2 Colonoscopy images. Erythematous colitis with punctate rash (similar to the rash on the mucosa of the upper GI tract). Ulcerative lesion of the mucosa of the sigmoid colon in the healing stage. Ongoing GI bleeding, the source is not detected.
During EGD and colonoscopy, it was not possible to detect the source of GI bleeding. According to the morphological analysis of biopsy specimens of the GI mucosa, GVHD were confirmed. The patient's condition worsened: an increase in the frequency and severity of gagging, an increase in the volume of the abdomen. Persistence of COVID-19 infection, then intestinal obstruction was detected by X-ray of the abdominal organs, an increase in inflammatory markers (CRP up to 19 mg/dL (norm is up to 0.5 mg/dL), PCT up to 16 ng/ml (norm is up to 0.5 ng/ml)).
In the screening culture from the oral cavity on the multi-resistant flora and fungi, the growth of yeast fungi Candida guilliermondii and mold fungi Rhizopus microsporus was detected. Based on the results of the minimum inhibitory concentration (MIC) for antimycotic drugs, caspofungin was discontinued and Ampholip 5 mg/kg/day intravenously was prescribed, oral treatment with amphotericin B was recommended Table 2.
Despite the ongoing therapy, the patient's condition continued to worsen due to the progression of the infection (further growth of inflammatory markers), dynamic intestinal obstruction, the appearance of signs of pancreatitis, ongoing GI bleeding, and an increasing of renal toxicity. For regenerative and immunomodulatory purposes, the patient underwent unrelated allogeneic bone marrow mesenchymal stem-cell transplantation and, for replacement purposes, granulocyte transfusion was performed (5 granulocyte transfusions for the entire time). However, there was no significant improvement in the patient’s general condition.
Due to the ongoing GI bleeding and in the absence of the effect of the ongoing replacement therapy, it was decided to repeat EGD and colonoscopy. According to the results, severe necrotizing pangastritis with the addition of a fungal infection was revealed. Moderate erythematous duodenopathy with lymphostasis and the addition of a fungal infection. Necrotizing colitis with total lesion of the ascending colon, focal lesion of the transverse colon, and sigmoid colon (Figure 3). A biopsy was performed.
Microbiological analysis of biopsy specimens of the stomach and the colon revealed the structures of mold fungi and yeast fungi: mold fungi- Rhizopus microsporus, resistant to all antimycotics used (Table 2) and yeast fungi-Candida lusitaniae, sensitive to micafungin, resistant to amphotericin B (including lipid form).
Isolated microorganisms |
Amount |
|||
1 |
Candida guilliermondii |
+ |
||
2 |
Rhizopus microsporus |
+ |
|
|
Antibiotics |
1 |
2 |
||
posaconazole |
0.064 |
32 |
||
micafungin |
0.25 |
32 |
||
Caspofungin |
32 |
|||
amphotericin |
S |
3 |
||
fluconazole |
S |
32 |
||
voriconazole |
S |
32 |
||
itraconazole |
S |
|
32 |
|
Microbiology laboratory note |
||||
MIC of the antimycotic drugs is in µg/ml |
||||
MIC of Isavuconazole is 32 µg/ml |
|
|||
Table 2 Minimum inhibitory concentration (MIC) for antimycotic drugs
Based on the data obtained, it was decided to add micafungin to the treatment at a dose of 100 mg/day, namely a combined antifungal therapy with micafungin in conjunction with the lipid form of amphotericin B (ampholip) was conducted.
Histological examination of biopsy specimens of the stomach, duodenum, ascending colon, and descending colon showed a similar morphological picture. The structure of the mucous membrane was completely replaced by granulation tissue with rare foci of coagulation necrosis with inflammatory purulent-granulomatous infiltration and numerous mycelium structures. Mycelium with thick walls, nonseptate, focal-branching, with bulbous extensions at the ends. Among the mycelium, scattered spores and rare conidia were determined (Figure 4, 5).
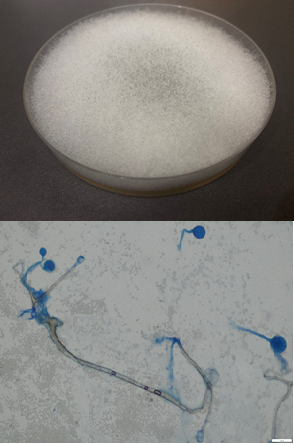
Figure 4 Growth of multiple colonies and elements of the micellar structure of Rhizopus microsporus: (a) growth on Subaro medium, 30°C, 4 days. (b) Visualization of a micropreparation of Rhizopus microsporus stained with blue lactophenol: rhizoids (1), sporangiophores (2), with sporangia (3), sporangiospores (4), x400 (Olympus BX 43F microscope, Japan).
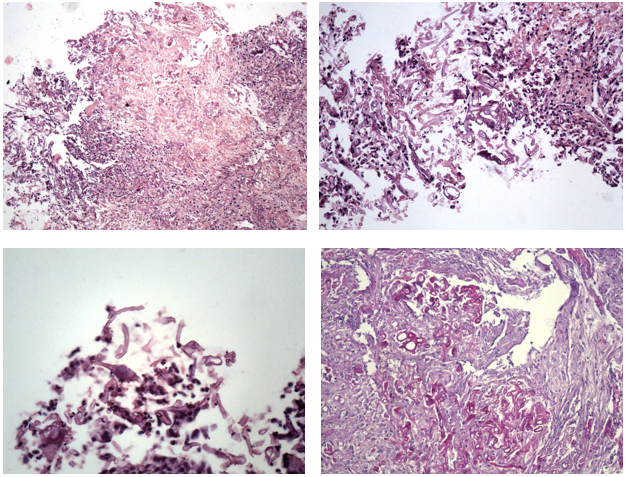
Figure 5 Fungal enterocolitis. (a) A focus of coagulation necrosis, surrounded by lymphocytes, fibroblasts, with numerous fragments of mycelium, hematoxylin-eosin, x50. (b) Fragments of unsepted mycelium of different thickness, branching at a right angle, hematoxylin-eosin, x200. (c) Conidia among mycelial structures, hematoxylin-eosin, x400. (d) Intense pink staining of mycelial structures, stained according to the Schiff test, x200.
For about two weeks, the patient received combination antifungal therapy (ampholip + micafungin), however, during the microbiological examination of fluid from the abdominal cavity, Candida lusitaniae was isolated (sensitivity: isavuconazole MIC-0.94 μg/ml, voriconazole MIC-0.38, micafungin MIC-1.0, itraconazole MIC-0.38, fluсonazole MIC-6.0, caspofungin MIC-3.0, posaconazole MIC-0.64, amphotericin B MIC-32.0-R-resistance according to EUCAST). Based on these results, posaconazole oral suspension via a nasogastric tube was added to the antifungal therapy.
The unpredictable absorption of posaconazole oral suspension, especially in patients with mucositis or neutropenic colitis during chemotherapy, requires controlling the residual serum concentration of posaconazole that is at present not possible in the Republic of Belarus.
Tablet and intravenous forms of the drug are not registered in the Republic of Belarus, therefore, after 2 weeks, it was decided to replace the posaconazole oral suspension with voriconazole intravenously (8 mg/kg/day).
Despite the ongoing massive combined antifungal therapy (three drugs at the same time), generalization of the infection with a total lesion of the GI tract occurred.
Invasive mucormycosis can be successfully treated only by surgery removing the affected tissues that was not possible, given the prevalence of the process and absolute contraindications for surgery due to the severity of the condition and the high risk to the patient's life in this case.
Despite ongoing treatment, generalization of the panresistant fungal infection, long-term persistent coronavirus, and GVHD did not allow the patient to cope with a severe infectious complication.
His condition worsened due to an increase in the syndrome of multiple organ failure (respiratory failure, kidney failure, liver failure, heart failure) and an infectious process. In dynamics, cardiotonic support, artificial lung ventilation were required, and in July 2022, the death of patient T was certified.
The total duration of the antifungal therapy was 218 days. The duration of the combined three-component antifungal therapy was 50 days.
In the etiological structure of micromycetes isolated from the respiratory organs and respiratory tract of children with oncohematological pathology, the proportion of mold microflora is 30.69% (85 out of 277 strains) with the predominance of representatives of the genus Aspergillus (69.41%-59 out of 85 strains).
The specific weight of the pathogens of mucormycosis in the form of Mucor spp., Rhizopus spp., Rhizomucor spp., and Lichtheimia [Absidia] corymbifera among mycelial microflora reaches 14.12% (12 out of 85 strains). Rhizopus microsporus is one of the fungal pathogens that cause the severe clinical course of mucormycosis. The reported case of invasive gastrointestinal mucormycosis against the background of an immunological disease demonstrates the possibility of an unfavorable outcome of a fungal infection of the GI tract against the background of a congenital pathology of the immune system in combination with a coronavirus infection after the hematopoietic stem cell transplantation.
None.
Authors declare that there is no conflict of interest.

©2023 Niafiodava, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.