Journal of
eISSN: 2373-437X


Research Article Volume 8 Issue 5
1Department of Chemistry, Faculty of Science, University of Kordofan, Sudan
2Phylum of Medical Parasitology, Medical Laboratory Sciences Department, Faculty of Health Science, Elsheikh Abdallah Elbadri University, Sudan
Correspondence: Mosab Nouraldein Mohammed Hamad, Phylum of Medical Parasitology, Medical Laboratory Sciences Department, Faculty of Health Science, Elsheikh Abdallah Elbadri University, Sudan, Tel +249929194137
Received: September 08, 2020 | Published: September 28, 2020
Citation: Hussein MB, Hamad MNM. Synthesis and antibacterial activity of 3-nitrobenzaldehyde semicarbazone ligand and its Ni (II) and Cu (II) Complexes. J Microbiol Exp. 2020;8(5):163-165. DOI: 10.15406/jmen.2020.08.00302
Schiff bases are the most widely used organic compounds. They have been shown to exhibit a broad range of biological activities, including; antifungal, antibacterial, anti-malarial, anti-proliferative, anti-inflammatory, antiviral, and antipyretic properties. In this study semicarbazone ligand was prepared by condensing 3-nitropenzaldehyde with semicarbazide hydrochloride in 1:1 molar ratio in ethanolic medium. This ligand was used to synthesize metal complexes of copper (II) and nickel (II) in 1:2 molar ratio using ethanol as a solvent .Characterization and structure elucidation of prepared metal complexes have been investigated on the basis of molar conductance and UV and IR spectral studies. The all prepared compounds showed a vital effect against both types of bacteria gram positive (Staphylococcus-aurous) and gram negative (Escherichia-coli).
Keywords: schiff bases, metal complexes, antibacterial activities
Schiff bases play important role in co-ordination chemistry as they easily form stable complexes with most transition metal ions. Many biologically important Schiff bases and their metal complexes have been reported in literature possessing, analytical, industrial, biological, clinical, biochemical, antimicrobial, anticancer, antibacterial, antifungal and antitumor activity1,2 in addition with important roles in ranging from anticorrosion, soil treatment agents and medicinal agents.3,4 Schiff base ligands and their transition metal complexes have been extensively investigated due to their wide range of applications including catalysts, medicine, crystal engineering, anticorrosion agent.5,6 Schiff bases are widely studied due to their synthetic flexibility, selectivity and sensitivity towards the central metal atom, structural similarities with natural biological compounds and also due to presence of azomethine group (-N=CH-)which imports in elucidating the mechanism of transformation and racemization reaction biologically. Schiff bases having chelation with oxygen, nitrogen etc. Imine or azomethine groups are present in various natural, derived and non natural compounds. The imine group present in such compounds has been shown to be critical to their biological activities.7 Schiff base complexes containing two or more metal centres are efficient catalysts. It is also well known that coordination of a ligand to metal ion acts synergistically to increase the biological activity of the ligand and decreases the cytotoxic effects of metal ion and ligand.8 This study concentrates on the synthesis and biological activity of Schiff base and its metal complexes.
Chemicals and reagents
Semicarbazidehydrochloride, Nickel chloride, Copper chloride dihydrate, Sodiumhydroxide (CDH), 3-nitrobenzaldehyde (FSA), Absolute ethanol (GCC).
Synthesis of ligand
(0.01mol, 1.11g) of semicabazide hydrochloride and (0.01mol, 0.4g) of Sodium hydroxide were dissolve in (20ml) of a hot ethanol and (0.01mol, 1.51g) of 3-nitro benzaldehyde was dissolve in (20ml) of ahot ethanol and shake well with constant stirring at 78°C for 7hrs.Yellow precipitate separated out filtered and dried Scheme 1.
Synthesis of complexes
Substituted ligand (0.0002mol, 0.042g) was dissolved in (20ml) of ethanol and (0.0001mol) of metal salts Cu(II) or Ni(II) in (20ml) of warm ethanol was added to the previous solution with stirring for 3hrs. The reaction mixture was allowed to stand and then filtered and dried. Reaction scheme was shown Figure 1.
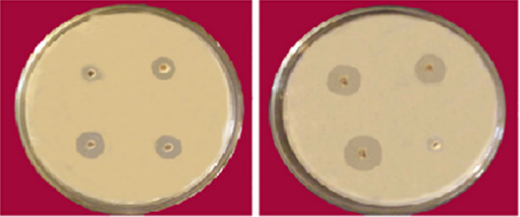
Figure 2 Antibacterial bacterial activity of Semicarbazone Cu (II) and Ni (II) complexes against E. coli.
IR spectral analysis
The appropriate weight of the ligand and their complexes was record and grinding with potassium bromide to form disk out let for the infrared (IR) spectrum record within the range 500-4000cm-1.
UV-Vis spectral analysis
A solutions with a concentration of (1×10-3 mol.L-) for the ligand and (1×10-5 mol.L-) for the complexes were prepared. The absorbance spectra were recorded in the visible and ultraviolet region with 200-800 nm using ethanol as a reference solution.
Conductivity measurement
Attended solutions concentration of (2×10-3 mol.L-) for the prepared complexes and measured the electrical conductivity.
Antimicrobial activity
Preparation of nutritious medium for the growth of bacteria
Take 28 grams of nutrient agar and dissolve in 1000 ml of distilled water, then set at the water bath at 45°C until the solution is homogenized and sterilized in autoclave at 121°C for 15-20 minutes Press 15 psi and leave the solution to cool down to 47°C and then pour in Petri dishes.
Preparation of the blood nourishing medium for bacterial growth
After leaving the solution to the blood stream.
Method of working fossils known diameters
The craters worked on the surface of the nutrient medium in Petri dishes, which grew in the bacterial colonies and the hole diameter of 8 mm with a hole in the center of the dish filled with absolute alcohol for methanol or distilled water for control. Measurements were made for the diameters of the extract, which were poured into holes through the diaphragm (Verna). The calculations were carried out to determine the extent of the spread of the extract on the bacterial colonies, "diameter of the extract-diameter of the hole" and the results were recorded between the ligand and metal ion. The complexes were dissolved in ethanol and the results showed that the complexes exhibit high melting points, indicating a strong bonding the height conductance values of the complexes support their electrolytic nature of the compounds to cool down to 47°C, add 2.5 ml fresh human blood and mix well, then pour on clean and sterile Petri dishes.
Physical properties of ligand and its complexes
The ligand and its complexes are stables in air they have varied colors, and the all prepared compounds are soluble in ethanol. Melting points were measured using open capillary tubes on melting point apparatus and the molar conductivity of (2×10-3 mol.L-) of their solution was measured at room temperature (Table 1).
Compound |
M. wt |
Colour |
Appearance |
Yield % |
m.p°C |
Molar Conductance (Ω-1cm2mol-1) |
L,sub>1 |
208 |
Yellowish |
Powder |
73.5 |
249 |
ـ |
[Cu(L)2]Cl2 |
549 |
Yellow |
Crystal |
57.6 |
252 |
119 |
[Ni(L)2]Cl2 |
544 |
Orange |
Crystal |
50 |
265 |
147.8 |
Table 1 Physical properties of ligand and its complexes
L=C8H8O3N4, M.wt=Molecular weight, m.p=melting point
Electronic spectral data of ligand and its complexes
Electronic spectra of complexes have band at (312-313 nm) assignable to π–π⃰ .9
IR data of ligand and its complexes
The IR spectrum of the prepared compounds showed absorption peaks at1683-1685cm-1 due to the stretching of the conjugate C=N10 and the appearance of absorption peak at 1716cm-1 due to the expansion of the association C=O and note that the decrease of this absorption in the complex, indicating the metal bonding oxygen11 and the appearance of absorption peak at 3161cm-1 due to NH the reduction of the absorption in the complex, indicating the association of the metal with the nitrogen and the emergence of absorption in the region 3464cm-1 is due to the NH2 group12 and the appearance of absorption peaks in the region 522-630cm-1 and 451-563cm-1 due to the metal bonding with oxygen and nitrogen respectively (table 2).13
Compound |
u(NH2) |
u(NH) |
u(C=N) |
u(C=O) |
u(M-O) |
u(M-N) |
L1 |
3464m |
3161m |
1683m |
1716s |
- |
- |
[Cu(L1)2]Cl2 |
3464m |
3159m |
1685m |
1712s |
630w |
563w |
[Ni(L1)2]Cl2 |
3464m |
3159m |
1683m |
1712s |
522w |
451w |
Table 2 IR and UV-Vis spectral data of ligand and its complexes
L= ligand, m = medium, s = strong, w = weak
Compound |
Concentration |
Diameter of inhibition zone (mm) |
|
Escherichia-coli |
Staphylococcus-aurous |
||
Ligand (L) |
(2×10-3 mol.L-) |
9 |
8 |
[Cu(L)2]Cl2 |
(2×10-3 mol.L-) |
13 |
10 |
[Ni(L)2]Cl2 |
(2×10-3 mol.L-) |
16 |
13 |
Table 3 Antibacterial activity of ligand and its metal complexes
Antibacterial activity
The synthesized ligand and its corresponding complexes were screened for their antibacterial activity against two types of bacteria Gram-negative (Escherichia-coli) and Gram-positive (Staphylococcus-aurous). As shown in table 3 the activity determined by using the Inhibition zone technique. All tests the organisms were growth on nutrient agar medium. Methanol was used as standard reference compound.
In This study we have synthesized biologically active semicarbazone ligand and it’s Cu (II) and Ni (II) complexes. The synthesized ligands and their derivatives were characterized and identified on the basis of physical and spectral data. Antibacterial activities were found that metal complexes are more active than the ligands that are indicated that the coordination increases their bioactivity. The more investigations are going on with this hope that some of these compounds may be used as antimicrobial agent.
The authors would like to express their gratitude to the Department of Chemistry, Faculty of Science, University of Khartoum, for providing research facilities.
Authors declare that there is no conflict of interest..

©2020 Hussein, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.