Journal of
eISSN: 2373-437X


Research Article Volume 10 Issue 1
1Department of Biotechnology, Flinders University, Australia
2Department of Microbiology, Tri Chandra Campus, Nepal
Correspondence: Sita Ram Sapkota, Department of Biotechnology, Flinders University, Adelaide, Australia , Tel +61431958850
Received: January 30, 2022 | Published: February 23, 2022
Citation: Sapkota SR, Paneru TP. Study the stability of chickpea endophytic actinobacteria species on broth and agar culture media. J Microbiol Exp. 2022;10(1):38-43. DOI: 10.15406/jmen.2022.10.00350
The actinobacteria acts by colonising plant roots and increases the nitrogen fixation capacity of the rhizobial partner. In this study, endophytic actinobacterial strains CP21A2, CP56, CP84B, and CP200B isolated from chickpea were evaluated for the sporulation rate in solid and liquid media. These resultant spores were evaluated for their stability at different pH and temperature. Calcium carbonate in the liquid broth and MS medium in solid agar media can be used to increase the sporulation rate of the actinobacteria. Additionally, we found out that almost all spore-producing strains were stable at 70°C 4 minutes but temperatures greater than that were lethal to the spores obtained from both types of media. In addition, the tested spore strains were more sensitive and prone to lysis at alkaline pH rather than acidic. Furthermore, our study suggested that CP56 spores obtained from liquid media and CP84B from solid media can be the best performers in promoting the overall growth of plants and nodules. However, further detailed investigations need to be carried out in order to determine their influence on the growth and development of legume plants which can be useful to increase the yield in the agricultural industry.
Actinomycetes, also known as actinobacteria, are gram-positive fungus-like filamentous bacteria normally found in soil. These bacteria have high G+C content in their DNA, many of which are filamentous with substrate and aerial mycelia. Approximately 22,000 bioactive secondary metabolites of microbial origin have been reported so far, of which fifty percent are from actinobacteria only.1 Approximately 160 antibiotics are being currently used in human therapy and in agriculture2 from actinobacteria. Liquid medium is preferred over solid medium for the production of spore because of its rapidity and is less expensive than the solid medium. However, there have not been any reports till date. Therefore, in the present study some experiments were carried out with a higher inoculation rate to enhance longer time for the sporulation on the plates as well as to determine the maximal number of spores that could be added to one 9 cm diameter petri plate. Further some experiments were carried out in order to understand whether temperature, size of inoculum, and incubation time helps with the processes of sporulation on the agar medium and/or either the presence of mycelium. The previous study inline that calcium chloride and humic acid are related with the most effective in increasing sporulation. Therefore, more research is required and drastic improvements has to be made in liquid and solid media for actinomycetes to increase sporulation. Further, the sporulation of these kinds of bacteria is studied in surface grown cultures, research on sporulatuion in submerged cultures is considered to be an attractive alternative. The culturing time is much shorter, synchronous sporulation can be more readily achieved with spores maturing over a subsequent period of 10-12 h. with high yields under controlled sterile conditions as well as a simpler scale-up .Therefore, more research is required and drastic improvements has to be made in liquid and solid media for actinomycetes to increase sporulation. The symbiotic system between legumes and rhizobia always attracts the interest of BNF researchers because of the greatest impact on the cycle of nitrogen. Fixed nitrogen is the nitrogen gas which is transformed to nitrate, an ammonium ion, ammonia, or another nitrogen oxide, from which living organisms could use as a nutrient.3,4 Excessive nitrogen fertilizer application could result in some environmental problems such as acid rain, the greenhouse effect and eutrophication.
To reduce the negative and unpredictable impacts, biological nitrogen fixation (BNF) is used as an alternative option for chemical fertilisers.5 The legumes and rhizobia symbiosis process can be outline according to the main steps: rhizobia attach to the root tip and enter via the root hairs causing hair curling, infection pocket, infection thread formation, nodule initiation, nodule formation and nitrogen fixation.6 Chickpea is currently the second most important pulse crop produced in the world. It is a rich source of protein for human consumption and is cheaper compared to animal protein. According to FAO statistics,7 global chickpea production was estimated at 13.7 million tonnes in 2014 with around 14 million hectares under chickpea cultivation. Chickpea is a type of legume which can convert atmospheric nitrogen via symbiosis with the rhizobial partner, Mesorhizobium.6
Actinobacteria are a kind of Gram-stain positive bacteria that have a high guanine and cytosine content in their genome. Actinobacteria can produce a slenderer and non-septate mycelium although some genera are unicellular like bacteria. They can be found in freshwater, soil and support the degradation of organic substances degradation (such as chitin and organic acids, protein fats, polysaccharides and cellulose). Therefore, they play an important role in turnover of organic matter, carbon cycle and humus formation, providing the nutrients into the soil. They can survive in a broad variety of soil type from high to low pH.8 Endophytic actinobacteria are bacteria that inhabit the inside of the plant without causing any visible negative changes to their plant hosts. They could protect the host plants from diseases and insects. They are not only found in the rhizosphere but also inside plants. Frankia is an actinobacterial genus found in association with pine trees that form nodules which can fix nitrogen.9 The endophytic actinobacteria include Streptomyces, Glycomyces Plantactinospora, Streptosporangium, Promicromonospora, Actinomadura, Kibdelosporangium, Nocardioides, Pseudonocardia, Actinopolyspora, Microbispora, Brevibacterium, Kitasatospora, Polymorphospora, Micromonospora, and Nocardia that could be found in various plants.8
The growth, nodulation process and nitrogen fixation of the host leguminous plants as well as the properties of the rhizobial strain may be influenced by compounds and phytohormones that are released by endophytic actinobacteria either directly or indirectly. Because the auxin balance is important for the improvement of nodule formation and the root plant development, the IAA released from actinobacteria is a necessary factor to enhance the plant growth and nodulation as well as the growth of co-inoculated rhizobia.10 The spores are traditional endospores with all the properties of bacterial endospores, ultrastructure, and physiology. Beside the mycelial development, spore arrangement is the most significant morphological basis that can be utilized to perceive an actinobacterium. Routinely, the arrangement of spores is confined to the morphological gathering of sporoactinomycetes, where sporulation happens in very much characterized pieces of the mycelium. It is realized that various qualities are associated with spore development and that diverse development conditions can have an impact on the spore arrangement.11 In general, spore production is optimal when the actinobacteria are cultured on a solid surface such as agar or a grain such as barley or soybean. However, scale up is a challenge as a large surface area is required and the spores have to be washed off the surface. Spore production is not common in liquid submerged culture as the actinobacteria grow as mycelium or pellets. However, if liquid media could be used for the actinobacteria spore production at a high titer it would be less expensive and faster, thereby improving the economics of the process. This project aims to optimize the medium for the production of spores in submerged culture. The spores produced in this way may have slightly different properties therefore a comparison with spores produced in the traditional manner will also be carried out to gauge their stability to changes in pH and temperature and, more importantly, their efficacy as inoculants.
Herein, we used the different strains of endophytic actinobacteria in order to evaluate their influence in chickpea plant which can be used as a promising source for the boosting the chickpea growth, nodulation, nitrogen fixation or soil borne disease bio-control agents for chickpea production.The overall aim of this research is to make improvements in liquid media in order to increase sporulation while keeping the media production cost low thus making the process economically viable. The spores produced in this manner will be compared for stability and efficacy against spores produced on agar.
The specific objectives of this research are to:
Organisms used
Chickpea seed (cv. Kabuli genesis 090) and the rhizobial culture Mesorhizobium ciceri CC1192 were provided by the South Australian research and development institute (SARDI) and endophytic actinobacteria Streptomyces sp. CP200B, CP84, CP21 and CP56 were obtained from previous research in our laboratory.
Spore production in liquid media
Galactose, at half the normal concentration, glutamic acid yeast extract medium (½ GGY) was used as liquid media (van Dissel et al., 2014). Initially, galactose (15 g), glutamic acid (1 g), yeast extract (5 g), anhydrous Iron (II) sulphate (0.001 g), anhydrous magnesium sulphate (0.25 g), and dipotassium hydrogen phosphate (0.2 g) was mixed in 1 liter of sterile water. Three flasks for each treatment, one for each strain, were prepared. Fifty microliter was transferred to each flask using 50 ml syringe and different concentrations of humic acid, calcium carbonate, calcium chlorides, and chitin were added to each flask and the content was sent to autoclave. After autoclaving, the medium was allowed to cool down and each treatment was inoculated with the 2 loopful of the cultures CP56, CP84B, CP200B, and CP21A2 in broth medium for 60 days. The inoculated medium was then placed on a 150-rpm shaker for 10 days at 27°C.
The spore counting was done via the method developed by Miles and Misra (MILES AND MISRA, 1938) Serial dilution of the endophytic actinobacterial spore suspension was prepared by adding 1x spore suspension and 9x diluent and dilutions were made up to 1012. Two drops of 10ul of suspension dilutions inoculated onto Mannitol Soy flour (MS) agar plates which were divided into 6 sectors. The plates were incubated at 27°C and the colonies were counted in each sector which had less than 10 colonies.12 Overgrowth was observed at the lower dilutions over the area of drop. The number of colony forming units per ml was calculated by using the following formula (Zhang et al., 2020). Collected spores were centrifuged at 3500 rpm for 5 minutes and the spores are stored in 20 % (v/v) glycerol at -20°C until further use.
CFU per ml = Average number of colonies for a dilution x dilution factor x 102.
Visualization of spore-bearing structures of isolated endophytic actinobacteria
The visualization of endophytic actinobacteria was done in a light microscope. Briefly, the actinobacteria was inoculated in 1/2 GGY media. One loop full of spores were added on slides and observed under a microscope.12
Preparation of inoculum
A loopful of four stains CP56, CP84B Cp200B and Cp21A2 in ½ Galactose-Glutamic Acid-Yeast extract liquid media were taken for inoculum. Culture was allowed to incubate at constant room temperature for 10 days at 150 rpm on a shaker. Culture was filtered using a sterile syringe half-filled with sterile cotton wool. The spores were counted by Miles and Misra technique. And the filtrate was centrifuged at 3500 rpm for 15 minutes. 4/5 of the total volume of the culture that has been spun down was removed. In this case, 30 ml was spun down and 24 ml was removed, leaving 6 ml of concentrated inoculum. The concentrated spores were resuspended in 8 ml 20 % (v/v) glycerol and stored.
The two media: Mannitol Soy flour agar (MS) and Mannitol soy flour oatmeal agar (MSO) were prepared a few days before the experiment and checked for contamination. Inoculum was prepared just before inoculation of the plates. Each treatment has 3 replicate plates. 0.1ml of inoculum was spread onto each plate. Plates were incubated at 27°C for 6 days. Plates were harvested by scraping off spore and mycelium lawn on the surface of the media. Replicates were stored in the same manner. Mannitol Yeast extract agar was not harvested as no spore lawn was formed despite visible mycelium growth. Harvested spores and mycelium were resuspended in 12 ml 20 % glycerol to inhibit growth and to protect the spores during freezing. Total volume of suspension for all tubes was 14 ml. Spore suspensions stored at -20°C. The colony forming units of endophytic actinobacteria spores were counted using the drop-plate technique described by Miles and Misra13 as mentioned previously (Hedges et al., 1978).
Stability of spores obtained from liquid broth and solid media
Actinobacteria spores from four different cultures (CP56, CP200B, Cp84B, and CP21A2) optimized in liquid and solid media were used for the stability testing. The different parameters used were temperature and pH.
Temperature
The stability studies were done at the different time interval at 70°C and 90°C. Initially, each strain obtained from liquid and solid media were diluted in sterile water to get 1.4 × 1010 CFU/ml. 10µl of each were added along with 4ml 0.9 % NaCl to each glass tube in triplicate. The sample was incubated at 70°C and 90°C separately. The spore counting was done after 1, 3, 6, 12, and 24 hours using Miles and Misra method as described previously.
pH
Stability testing of the solid and liquid spore was done in the three different pH (6.0, 7.0, and 8.0). Initially different pH was maintained in 0.9% NaCl by the addition of ammonium bicarbonate or 0.1N HCl. The dilution and spore count were added to each 4 ml 0.9% NaCl with different pH as mentioned previously.
The study of sporulation in submerged media presents a better alternative to sporulation on solid medium or surface growth medium for several reasons. First, scale up can be carried out to boost yields of the inoculant, which can give immense economical advantage. The submerged system also differentiates between genes responsible for the control of hyphae formation and the gene involved in sporulation. Therefore, submerged sporulation can be an excellent model system to study sporulation than a solid-state medium.
Data collection and analysis
The data was entered and collated in a MS Excel spreadsheet and subsequently analysed using the IBM SPSS Statistics version 25.0.
Spore production in liquid broth
Table 1 demonstrated the treatment of endophytic actinobacteria isolated from chickpea plants treated with 0.1% methyl jasmonate. It showed that methyl jasmonate increased the sporulation of CP84B strain significantly compared with other strains.
|
Culture strain |
Methyl jasmonate 0.1% |
½ GGY (control) |
|
CP56 |
1.6 × 1013 |
1.5 × 1013 |
|
CP200B |
1.4 × 1013 |
1.4 × 1013 |
|
CP84B |
5.5 × 1011 |
3 × 1011 |
|
CP21A2 |
3 × 1011 |
3 × 1011 |
Table 1 Endophytic actinobacteria isolated from chickpea plants treated with 0.1% methyl jasmonate. The spores were counted in CFU/ml units in triplicates
Optimizing the sporulation of actinobacteria in solid media
The production of spores is dependent on several factors such as, pH, temperature, medium composition, humidity and amount of starting inoculum. The focus of this study was to determine the best media composition for the growth and sporulation of the four cultures. As shown in Table 4, mannitol soy agar served as the best medium for the growth and production of spores of CP56 (1.2 × 1012 CFU/ml) and CP84B (1.2 × 1012 CFU/ml). On the contrary, mannitol soy oatmeal agar was found to be superior in the case of CP200B (2.7 × 1011 CFU/ml) and CP21A2 (2.7 × 1012 CFU/ml).
Microscopic observation of endophytic actinobacteria strain spores obtained from liquid and solid agar media
Microscopic representative images were taken from a light microscope as shown in figure 1. The images taken clearly showed the morphological differences in the spores cultured in solid and liquid media.
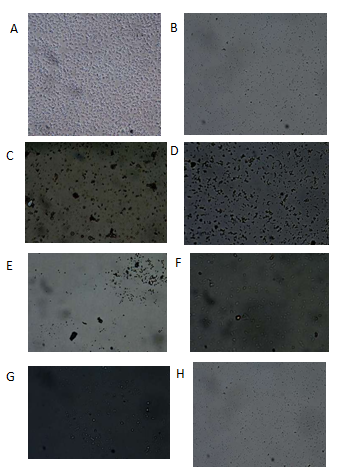
Figure 1 Entophytic actinobacteria strains A (CP56L), B (CP56S), C (CP84BL), D (CP84BS), E (CP200BS), F (CP200BL), G (CP21A2S), and H (CP21A2L) observed under 40X magnification with light microscope. S = solid; L = liquid.
Influence of temperature
The influence of temperature on the stability of spores of each of the 4 strains was exposed to various temperatures for up to 24 hours. The spores obtained from the different sources (liquid and solid media) responded differently in our study. For instance, when CP56 spores obtained from liquid and solid media sources were exposed to 70°C, the both types of spores were fairly stable until 12 h. However, after 24 h the spore from liquid broth showed exponential growth up to 0.5×108 CFU/ml but no spores from solid media were seen. In contrast, for the CP84B and CP21A2 strains, spores obtained from solid media were found to be more stable compared with the spore from liquid media. Further in the case of CP200B, spores from both media were found to be stable for a fair amount of time as evident by the data shown in Table 2. Further, most of the spores were found to be thermolabile after 6 h under exposure at 90°C. No germination or growth of the spores was found after 12 h under exposure at 90°C. Among the strains, CP56 from liquid media and CP21A2 from solid media were found to have more stable as 1.0×108 and 1.0×106 CFU/ml, respectively as shown in Table above.
|
Culture strains |
Mannitol Soy agar (CFU/per plate) |
Mannitol Soy Oatmeal agar (CFU/ per plate) |
|
CP56 |
1.2 × 1012 |
1.2 × 1011 |
|
CP84B |
1.2 × 1012 |
2.2 × 1011 |
|
CP200B |
1.2 × 1011 |
2.7 × 1011 |
|
CP21A2 |
1.7 × 1012 |
2.7 × 1012 |
Table 2 Comparison of sporulation in different agar media
Influence of pH
In order to determine the stability of the spores obtained from different strains, they were exposed to various pH (pH 6, pH 7, and pH 8). The results were shown in Table 3,4, and 5. while comparing the stability of spores in different pH, our study clearly showed that the spores are most stable at neutral pH. At pH 6.0, CP56 from liquid and CP84B from solid were found to be more stable than the other spores. In the case of CP56 and CP21A2, the spores obtained from liquid media were more stable than the solid ones. These spores were stable for more than 6 hrs.
|
Treatment time (Hrs) |
Strains |
|||||||
|
CP56 |
CP84B |
CP200B |
CP21A2 |
|||||
|
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
|
|
0 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
|
1 |
1.0×1010 |
1.0×1012 |
1.5×1010 |
1.0×1010 |
2.5×1010 |
2.0×1010 |
2.5×1010 |
1.5×1010 |
|
3 |
2.5×1010 |
1.0×1010 |
1.0×1010 |
2.5×108 |
2.0×1010 |
2.0×1010 |
2.5×108 |
1.5×1010 |
|
6 |
1.5×108 |
1.5×1010 |
1.5×108 |
1.5×1010 |
1.5×1010 |
2.5×108 |
0.5×108 |
1.5×1010 |
|
12 |
1.0×106 |
1.0×108 |
1.0×107 |
1.0×109 |
1.0×106 |
2.0×108 |
0.5×104 |
2.0×108 |
|
24 |
0.5×108 |
0 |
0 |
0.5×106 |
0.5×106 |
1.0×106 |
0 |
0.5×106 |
Table 3 Stability testing of different strains for temperature at 70°C
|
Treatment time (Hrs) |
Strains |
|||||||
|
CP56 |
CP84B |
CP200B |
CP21A2 |
|||||
|
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
|
|
0 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
|
1 |
1.5×1010 |
1.5×108 |
1.0×1010 |
1.5×1010 |
1.0×1010 |
1.0×108 |
2.5×108 |
1.5×1010 |
|
3 |
0.5×1010 |
0.5×106 |
0.5×1010 |
0.5×108 |
0 |
0.5×108 |
2.0×106 |
1.0×108 |
|
6 |
1.0×108 |
0 |
0 |
0 |
0 |
0 |
0 |
1.0×106 |
|
12 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
24 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Table 4 Stability testing of different strains for temperature at 90°C
0=No growth
|
Treatment time (Hrs) |
Strains |
|||||||
|
CP56 |
CP84B |
CP200B |
CP21A2 |
|||||
|
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
|
|
0 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
|
1 |
1.5×106 |
1.0×1010 |
2.0×108 |
1.2×1010 |
1.0×106 |
1.0×1010 |
1.0×1010 |
1.0×1010 |
|
3 |
2.5×104 |
0.5×108 |
1.0×106 |
1.0×108 |
0 |
2.0×108 |
1.0×108 |
0 |
|
6 |
1.5×104 |
1.0×106 |
0 |
1.0×106 |
0 |
0 |
1.0×106 |
0 |
|
12 |
0.5×104 |
0 |
0 |
1.0×104 |
0 |
0 |
0 |
0 |
|
24 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Table 5 Stability of different strains at pH 6
0=No growth
As expected, the spores obtained from both solid and liquid media for the 4 were stable until 24 hr of exposure under pH 7 as shown in Table 6. The CP56 strain spores from liquid media when exposed to pH 7, demonstrated fluctuation in the growth during the exposure in different time intervals. The number of spores increases after the exposure in pH 7 at 1 h (3×1010 CFU/ml), 3 h (2.0×1010 CFU/ml), and 6 h (2.5×1010 CFU/ml). Further exposure of spores for 12 h and 24 h, demonstrated decline in the number up to 1.0×108 and 1.0×106 CFU/ml, respectively. Similar trends were observed in the CP56 spores obtained from solid sources. Further, CP84B were found to be fairly stable until 12 h of exposure under pH 7. However, under 24 h of exposure spores from solid media were found to be more stable as evident by the number of spores 1×106 CFU/ml. Similarly, in the case of CP200B, the spores obtained from solid media were more stable than the liquid media. Interestingly, while exposing CP21A2 under pH 7, spores from liquid media were found to be more stable than the solid one. The number of spores was found to be higher than the spore before exposure until 6 h of exposure.
|
Treatment time (Hrs) |
Strains |
|||||||
|
CP56 |
CP84B |
CP200B |
CP21A2 |
|||||
|
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
|
|
0 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
|
1 |
3×1010 |
2.5×1014 |
2.0×1010 |
1.2×1012 |
2.0×1010 |
2.0×1014 |
1.4×1010 |
1.0×1012 |
|
3 |
2.0×1010 |
1.5×1012 |
1.0×1010 |
1.0×1012 |
2.0×1010 |
1.0×1012 |
1.0×1010 |
1.0×1010 |
|
6 |
2.5×1010 |
1.0×1012 |
1.0×108 |
1.0×1010 |
2.5×1010 |
1.0×1012 |
2.5×1010 |
1.0×108 |
|
12 |
1.0×108 |
1.5×1010 |
1.0×106 |
1.0×108 |
1.0×108 |
1.0×1010 |
0 |
0 |
|
24 |
1.0×106 |
0.5×106 |
0 |
1×106 |
0 |
0.5×106 |
1.0×104 |
0 |
Table 6 Stability testing of different strain at pH 7
0= No growth
The spores were further exposed to pH 8 for the duration of 24 hours. We found out that none of the spores from both sources were stable more than the 3 h of exposure except CP56 from liquid source as shown in Table 7. Further, no growth of the CP21A2 were found when exposed up to 3 h and is suggested that this spore from solid media was the more further, no growth of the CP21A2 were found when exposed upto 3 h and is suggested that this spore from solid media was the more prone to higher pH than the other spores.
|
Treatment time (Hrs) |
Strains |
|||||||
|
CP56 |
CP84B |
CP200B |
CP21A2 |
|||||
|
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
Liquid Spore (CFU/ml) |
Solid spore (CFU/ml) |
|
|
0 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
1.4×1010 |
|
1 |
1×1010 |
1.0×1012 |
1.0×108 |
1.0×106 |
1.0×1010 |
2.0×1010 |
1.0×1010 |
1.0×1010 |
|
3 |
1.0×106 |
1.0×108 |
1.0×106 |
1.0×104 |
1.0×106 |
1.0×104 |
1.0×104 |
0 |
|
6 |
1.0×104 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
12 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Table 7 Stability testing of different strain at pH 8
0= No growth
Previously, various agar media were used for the non-actinobacterial microorganism.14 YMA (a high nutrient media) was reported to have the highest level of non-actinobacteria. However, we used GGY media for our study because of the presence of galactose in the media. Galactose has been reported to enhance the growth rate of sporulation of actinobacterial strains. Previously, Rueda et al.,15 also reported that the sporulation rate was higher in the media containing galactose. Galactose was responsible for efficient sporulation and synthesis of glycogen. However, no spores or poor sporulation were obtained when used glucose, mannitol, fructose, and maltose instead.15
Further optimization of the four strains for sporulation on MS and MSO media were performed. The production of spores is dependent on several factors such as, pH, temperature, medium composition, humidity and amount of starting inoculum. The focus of this study was to determine the best media composition for the growth and cultures. As seen in Table above, mannitol soy agar served as the best media for the growth and production of spores of CP56 (1.2×1012 CFU/ml) and CP84B (1.2×1012 CFU/ml). On the contrary, mannitol soy oatmeal agar was found to be superior in the case of CP200B (2.7×1011 CFU/ml) and CP21A2 (2.7×1012 CFU/ml). The obtained results were concurrent with the previous reports published by Bennett et al. More importantly, the number of spores in our study were higher than the previous one which might be due to the different factors that include contamination, incubation time and temperature.16
Overall, endophytic actinobacteria strains CP21A2, CP56, CP84B, and CP200B isolated from chickpea were evaluated for the sporulation rate in solid and liquid agar media. These obtained spores were evaluated for their stability. Additionally, we found out that spores of almost all the 4 strains were stable at 70°C but temperatures greater than that are not suitable and lethal to the spores obtained from both media. In addition, the tested spore strains were more sensitive and prone to lysis in alkaline pH rather than acidic. However, liquid media offers shorter culturing time, with high yields under controlled sterile conditions as well as a simpler scale-up. The Major conclusion is that the hypothesis is validated as the spores produced in liquid are as stable and effective spores’ production on solid media.
Although this study has suggested that there is significant change in the morphology, stability, and influence of these spores from liquid and solid media, further detailed micromorphological investigation must be done. Further, the number of strains should be increased to validate our findings. Different soil containing complex microflora and natural rhizobial communities can be used in order to stimulate the growth and development of chickpea plant and other lentils in further investigation.
None.
Authors declare that there is no conflict of interest.

©2022 Sapkota, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.